Nanomedicine: Nanotechnology, Biology and Medicine ( IF 4.2 ) Pub Date : 2020-07-03 , DOI: 10.1016/j.nano.2020.102260 Sven Schnichels 1 , José Hurst 1 , Jan Willem de Vries 1 , Sami Ullah 1 , Agnieszka Gruszka 1 , Minseok Kwak 2 , Marina Löscher 1 , Sascha Dammeier 3 , Karl-Ulrich Bartz-Schmidt 1 , Martin S Spitzer 4 , Andreas Herrmann 5
|
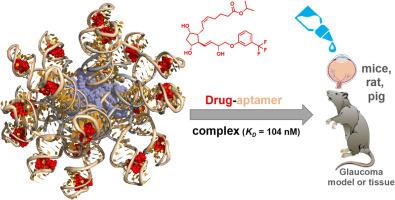
Lipid DNA nanoparticles (NPs) exhibit an intrinsic affinity to the ocular surface and can be loaded by hybridization with fluorophore-DNA conjugates or with the anti-glaucoma drug travoprost by hybridizing an aptamer that binds the medication. In the travoprost-loaded NPs (Trav-NPs), the drug is bound by specific, non-covalent interactions, not requiring any chemical modification of the active pharmaceutical ingredient. Fluorescently labeled Trav-NPs show a long-lasting adherence to the eye, up to sixty minutes after eye drop instillation. Biosafety of the Trav-NPs was proved and in vivo. Ex vivo and in vivo quantification of travoprost via LC–MS revealed that Trav-NPs deliver at least twice the amount of the drug at every time-point investigated compared to the pristine drug. The data successfully show the applicability of a DNA-based drug delivery system in the field of ophthalmology for the treatment of a major retinal eye disease, i.e. glaucoma.
中文翻译:

自组装的DNA纳米颗粒装载了travoprost用于青光眼治疗。
脂质DNA纳米颗粒(NPs)对眼表具有内在亲和力,可通过与荧光团DNA结合物或抗青光眼药物托沃前列素杂交(通过与药物结合的适体杂交)进行装载。在载有Travoprost的NP(Trav-NP)中,药物通过特定的非共价相互作用结合,不需要对活性药物成分进行任何化学修饰。荧光标记的Trav-NP在滴眼液后60分钟内对眼睛具有持久的附着力。Trav-NPs的生物安全性已得到证实并且在体内。通过的体内和体外定量travoprostLC-MS揭示,与原始药物相比,Trav-NP在每个研究的时间点上所输送的药物量至少是其两倍。数据成功地表明了基于DNA的药物递送系统在眼科领域用于治疗主要的视网膜眼病(即青光眼)的适用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号