当前位置:
X-MOL 学术
›
Nano Today
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational modeling on mitochondrial channel nanotoxicity
Nano Today ( IF 13.2 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.nantod.2020.100913 Michael González-Durruthy , Amal Kanta Giri , Irina Moreira , Riccardo Concu , André Melo , Juan M. Ruso , M. Natália D.S. Cordeiro
Nano Today ( IF 13.2 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.nantod.2020.100913 Michael González-Durruthy , Amal Kanta Giri , Irina Moreira , Riccardo Concu , André Melo , Juan M. Ruso , M. Natália D.S. Cordeiro
|
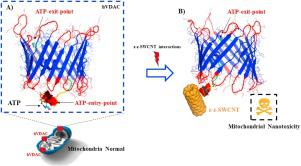
Abstract Herein, we evaluated the interactions between the zig-zag-single-walled carbon nanotube (z-z-SWCNT (8.0)) and the ATP-entry-point of the human mitochondrial voltage-dependent anion-selective channel (hVDAC1). For this purpose, both molecular docking and molecular dynamics simulations were performed. The flexibility properties of the referred ATP-entry-point was efficiently modeled using crystallographic validation-based on Ramachandran plot. The preferred conformations obtained for this segment were able to establish very favorable interactions with the ligands (ATP and z-z-SWCNT). Next, using both molecular docking and molecular dynamics simulations, we demonstrated that z-z-SWCNT can directly prevent the ATP-transition from its first entry-point residue (MET1). We suggested that the associated z-z-SWCNT aggregation can be responsible by avoiding the natural biochemical steps for the ATP-transport, according to a nanotoxicity mechanism based on hydrophobic interactions. The docking free energy of z-z-SWCNT/hVDAC1 and ATP/hVDAC1 complexes was remarkably close, according to local perturbation maps of the catalytic residues’ cluster (i.e. MET1, ARG2, GLY3, SER4, ALA5). On the other hand, the results of molecular dynamics simulations match the ones of the docking simulations, reinforcing the hVDAC1 channel nanotoxicity hypothesis. Overall, the obtained results could open new opportunities towards the rational design of new carbon nanomaterials and in silico mitotarget drug-discovery.
中文翻译:

线粒体通道纳米毒性的计算模型
摘要在此,我们评估了锯齿形单壁碳纳米管 (zz-SWCNT (8.0)) 与人类线粒体电压依赖性阴离子选择性通道 (hVDAC1) 的 ATP 入口点之间的相互作用。为此,进行了分子对接和分子动力学模拟。使用基于拉马钱德兰图的晶体学验证有效地模拟了所提及的 ATP 入口点的灵活性特性。为该片段获得的优选构象能够与配体(ATP 和 zz-SWCNT)建立非常有利的相互作用。接下来,使用分子对接和分子动力学模拟,我们证明了 zz-SWCNT 可以直接阻止 ATP 转变从其第一个入口点残基 (MET1)。根据基于疏水相互作用的纳米毒性机制,我们建议相关的 zz-SWCNT 聚集可以通过避免 ATP 运输的自然生化步骤来负责。根据催化残基簇(即MET1、ARG2、GLY3、SER4、ALA5)的局部扰动图,zz-SWCNT/hVDAC1 和ATP/hVDAC1 复合物的对接自由能非常接近。另一方面,分子动力学模拟的结果与对接模拟的结果相匹配,加强了 hVDAC1 通道纳米毒性假设。总体而言,所获得的结果可以为合理设计新的碳纳米材料和在 silico mitotarget 药物发现方面开辟新的机会。根据基于疏水相互作用的纳米毒性机制。根据催化残基簇(即MET1、ARG2、GLY3、SER4、ALA5)的局部扰动图,zz-SWCNT/hVDAC1 和ATP/hVDAC1 复合物的对接自由能非常接近。另一方面,分子动力学模拟的结果与对接模拟的结果相匹配,加强了 hVDAC1 通道纳米毒性假设。总体而言,所获得的结果可以为合理设计新的碳纳米材料和在 silico mitotarget 药物发现方面开辟新的机会。根据基于疏水相互作用的纳米毒性机制。根据催化残基簇(即MET1、ARG2、GLY3、SER4、ALA5)的局部扰动图,zz-SWCNT/hVDAC1 和ATP/hVDAC1 复合物的对接自由能非常接近。另一方面,分子动力学模拟的结果与对接模拟的结果相匹配,加强了 hVDAC1 通道纳米毒性假设。总体而言,所获得的结果可以为合理设计新的碳纳米材料和在 silico mitotarget 药物发现方面开辟新的机会。分子动力学模拟的结果与对接模拟的结果相匹配,加强了 hVDAC1 通道纳米毒性假设。总体而言,所获得的结果可以为合理设计新的碳纳米材料和在 silico mitotarget 药物发现方面开辟新的机会。分子动力学模拟的结果与对接模拟的结果相匹配,加强了 hVDAC1 通道纳米毒性假设。总体而言,所获得的结果可以为合理设计新的碳纳米材料和在 silico mitotarget 药物发现方面开辟新的机会。
更新日期:2020-10-01
中文翻译:

线粒体通道纳米毒性的计算模型
摘要在此,我们评估了锯齿形单壁碳纳米管 (zz-SWCNT (8.0)) 与人类线粒体电压依赖性阴离子选择性通道 (hVDAC1) 的 ATP 入口点之间的相互作用。为此,进行了分子对接和分子动力学模拟。使用基于拉马钱德兰图的晶体学验证有效地模拟了所提及的 ATP 入口点的灵活性特性。为该片段获得的优选构象能够与配体(ATP 和 zz-SWCNT)建立非常有利的相互作用。接下来,使用分子对接和分子动力学模拟,我们证明了 zz-SWCNT 可以直接阻止 ATP 转变从其第一个入口点残基 (MET1)。根据基于疏水相互作用的纳米毒性机制,我们建议相关的 zz-SWCNT 聚集可以通过避免 ATP 运输的自然生化步骤来负责。根据催化残基簇(即MET1、ARG2、GLY3、SER4、ALA5)的局部扰动图,zz-SWCNT/hVDAC1 和ATP/hVDAC1 复合物的对接自由能非常接近。另一方面,分子动力学模拟的结果与对接模拟的结果相匹配,加强了 hVDAC1 通道纳米毒性假设。总体而言,所获得的结果可以为合理设计新的碳纳米材料和在 silico mitotarget 药物发现方面开辟新的机会。根据基于疏水相互作用的纳米毒性机制。根据催化残基簇(即MET1、ARG2、GLY3、SER4、ALA5)的局部扰动图,zz-SWCNT/hVDAC1 和ATP/hVDAC1 复合物的对接自由能非常接近。另一方面,分子动力学模拟的结果与对接模拟的结果相匹配,加强了 hVDAC1 通道纳米毒性假设。总体而言,所获得的结果可以为合理设计新的碳纳米材料和在 silico mitotarget 药物发现方面开辟新的机会。根据基于疏水相互作用的纳米毒性机制。根据催化残基簇(即MET1、ARG2、GLY3、SER4、ALA5)的局部扰动图,zz-SWCNT/hVDAC1 和ATP/hVDAC1 复合物的对接自由能非常接近。另一方面,分子动力学模拟的结果与对接模拟的结果相匹配,加强了 hVDAC1 通道纳米毒性假设。总体而言,所获得的结果可以为合理设计新的碳纳米材料和在 silico mitotarget 药物发现方面开辟新的机会。分子动力学模拟的结果与对接模拟的结果相匹配,加强了 hVDAC1 通道纳米毒性假设。总体而言,所获得的结果可以为合理设计新的碳纳米材料和在 silico mitotarget 药物发现方面开辟新的机会。分子动力学模拟的结果与对接模拟的结果相匹配,加强了 hVDAC1 通道纳米毒性假设。总体而言,所获得的结果可以为合理设计新的碳纳米材料和在 silico mitotarget 药物发现方面开辟新的机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号