Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-07-03 , DOI: 10.1016/j.jmb.2020.06.026 Abhishek Narayan 1 , Soundhararajan Gopi 2 , Bincy Lukose 2 , Athi N Naganathan 2
|
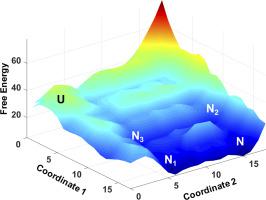
Paralogous proteins play a vital role in evolutionary adaptation of organisms and species divergence. One outstanding question is the molecular basis for how folding mechanisms differ in paralogs that not only exhibit similar topologies but also evolve under near-identical selection pressures. Here, we address this question by studying a paralogous protein pair from enterobacteria, Hha and Cnu, combining experiments, simulations and statistical modeling. We find that Hha is less stable and folds an order of magnitude slower than Cnu despite similar packing and topological features. Differences in surface charge–charge interactions, however, promote a N-terminal biased unfolding mechanism in Hha unlike Cnu that unfolds via the C terminus. Our work highlights how electrostatic frustration contributes to the population of heterogeneous native ensembles in paralogs and the avenues through which evolutionary topological constraints could be overcome by modulating charge–charge interactions.
中文翻译:

静电挫折形状折叠细菌压力响应蛋白中的折叠机制差异。
旁系蛋白在生物体的进化适应和物种差异中起着至关重要的作用。一个悬而未决的问题是,在旁系同源物中折叠机制如何不同的分子基础,该旁系同源物不仅表现出相似的拓扑结构,而且还在接近相同的选择压力下进化。在这里,我们通过结合实验,模拟和统计模型研究肠道细菌,Hha和Cnu的旁源蛋白对来解决这个问题。我们发现,尽管具有类似的堆积和拓扑特征,但Hha的稳定性较差,折叠速度比Cnu慢一个数量级。然而,表面电荷与电荷相互作用的差异促进了Hha中N端偏向的展开机制,而Cnu则通过C总站。我们的工作强调了静电挫败是如何促进旁系同源物中异质原生群体的聚集以及通过调节电荷-电荷相互作用可以克服进化拓扑约束的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号