Journal of Molecular and Cellular Cardiology ( IF 5 ) Pub Date : 2020-07-05 , DOI: 10.1016/j.yjmcc.2020.06.010 Matthew D Martens 1 , Jared T Field 1 , Nivedita Seshadri 2 , Chelsea Day 3 , Donald Chapman 4 , Richard Keijzer 5 , Christine A Doucette 2 , Grant M Hatch 6 , Adrian R West 3 , Tammy L Ivanco 7 , Joseph W Gordon 8
|
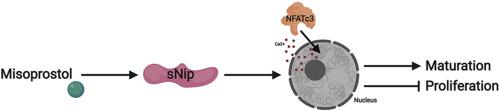
Systemic hypoxia resulting from preterm birth, altered lung development, and cyanotic congenital heart disease is known to impede the regulatory and developmental pathways in the neonatal heart. While the molecular mechanisms are still unknown, hypoxia induces aberrant cardiomyocyte proliferation, which may be initially adaptive, but can ultimately program the heart to fail in early life. Recent evidence suggests that the prostaglandin E1 analogue, misoprostol, is cytoprotective in the hypoxia-exposed neonatal heart by impacting alternative splicing of the Bcl-2 family member Bnip3, resulting in the generation of a variant lacking the third exon (Bnip3ΔExon3 or small Nip; sNip). Using a rodent model of neonatal hypoxia, in combination with rat primary neonatal cardiomyocytes (PVNCs) and H9c2 cells, we sought to determine if misoprostol can prevent cardiomyocyte proliferation and what the key molecular mechanisms might be in this pathway. In PVNCs, exposure to 10% oxygen induced myocyte proliferation concurrent with molecular markers of cell-cycle progression, such as Cyclin-D1, which were prevented by misoprostol treatment. Furthermore, we describe a critical role for sNip in opposing cardiomyocyte proliferation through several mechanisms, including reduced expression of the proliferative MEF2C-myocardin-BMP10 pathway, accumulation of nuclear calcium leading to NFATc3 activation, and increased expression of the cardiac maturation factor BMP2. Intriguingly, misoprostol and sNip inhibited hypoxia-induced glycolytic flux, which directly influenced myocyte proliferation. These observations were further supported by knockdown studies, where hypoxia-induced cardiomyocyte proliferation is restored in misoprostol-treated cells by an siRNA targeting sNip. Finally, in postnatal day (PND)-10 rat pups exposed to hypoxia, we observed histological evidence of increased nuclei number and increased PPH3 staining, which were completely attenuated by misoprostol treatment. Collectively, this data demonstrates how neonatal cardiomyocyte proliferation can be pharmacologically modulated by misoprostol treatment, which may have important implications for both neonatal and regenerative medicine.
中文翻译:

米索前列醇通过Bnip3,核周钙信号传导和糖酵解抑制作用减弱新生儿心肌细胞的增殖。
由早产,肺发育改变和先天性紫性心脏病引起的系统性缺氧会阻碍新生儿心脏的调节和发育途径。尽管分子机制仍然未知,但缺氧会诱导异常的心肌细胞增殖,这种增殖最初可能是适应性的,但最终可能使心脏编程,使其在早期生命中衰竭。最近的证据表明,前列腺素E1类似物,米索前列醇,是在缺氧暴露的新生儿心脏细胞保护由撞击Bcl-2家族成员的BNIP3选择性剪接,导致缺乏3外显子的变体的产生(BNIP3 ΔExon3或小Nip;sNip)。使用啮齿类动物的新生儿缺氧模型,结合大鼠原代新生儿心肌细胞(PVNC)和H9c2细胞,我们试图确定米索前列醇是否可以预防心肌细胞增殖以及该途径中的关键分子机制可能是什么。在PVNC中,暴露于10%的氧气会诱导心肌细胞增殖,并伴随着细胞周期进程的分子标志物(如Cyclin-D1)被米索前列醇治疗所阻止。此外,我们通过多种机制描述了sNip在对抗心肌细胞增殖中的关键作用,包括减少增殖性MEF2C-心肌素-BMP10途径的表达,导致NFATc3活化的核钙蓄积以及心脏成熟因子BMP2的表达增加。有趣的是 米索前列醇和sNip抑制缺氧诱导的糖酵解通量,直接影响心肌细胞的增殖。击倒研究进一步支持了这些观察,其中低氧诱导的心肌细胞增殖通过靶向sNip的siRNA在米索前列醇处理的细胞中得以恢复。最后,在暴露于低氧的出生后第10天(PND)的幼崽中,我们观察到组织数目增加的核数和PPH3染色的组织学证据,这些结果被米索前列醇治疗完全减弱。总的来说,该数据表明米索前列醇治疗如何通过药理调节新生儿心肌细胞的增殖,这可能对新生儿医学和再生医学都有重要意义。击倒研究进一步支持了这些观察,其中低氧诱导的心肌细胞增殖通过靶向sNip的siRNA在米索前列醇处理的细胞中得以恢复。最后,在暴露于低氧的出生后第10天(PND)的幼崽中,我们观察到组织数目增加的核数和PPH3染色的组织学证据,这些结果被米索前列醇治疗完全减弱。总的来说,该数据表明米索前列醇治疗如何通过药理调节新生儿心肌细胞的增殖,这可能对新生儿医学和再生医学都有重要意义。击倒研究进一步支持了这些观察结果,其中低氧诱导的心肌细胞增殖通过靶向sNip的siRNA在米索前列醇处理的细胞中得以恢复。最后,在暴露于低氧的出生后第10天(PND)的幼崽中,我们观察到组织数目增加的核数目和PPH3染色的组织学证据,这些结果被米索前列醇治疗完全减弱。总的来说,该数据表明米索前列醇治疗如何通过药理调节新生儿心肌细胞的增殖,这可能对新生儿医学和再生医学都有重要意义。我们观察到组织学证据表明核数目增加和PPH3染色增加,而米索前列醇治疗可完全减弱这种染色。总的来说,该数据表明米索前列醇治疗如何通过药理调节新生儿心肌细胞的增殖,这可能对新生儿医学和再生医学都有重要意义。我们观察到组织学证据增加了核数目和PPH3染色增加,而米索前列醇治疗完全减弱了这种染色。总的来说,该数据表明米索前列醇治疗如何通过药理调节新生儿心肌细胞的增殖,这可能对新生儿医学和再生医学都有重要意义。



























 京公网安备 11010802027423号
京公网安备 11010802027423号