Environmental Research ( IF 7.7 ) Pub Date : 2020-07-05 , DOI: 10.1016/j.envres.2020.109865 S Keerthanan 1 , Suranga M Rajapaksha 2 , Lukáš Trakal 3 , Meththika Vithanage 1
|
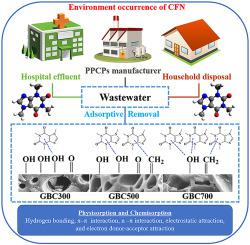
The present study aimed to envisage the effect of physicochemical properties on the performance of Gliricidia sepium biochar (GBC) pyrolyzed at 300, 500, and 700 °C in the removal caffeine (CFN); a pharmaceutical and personal care product, from water. The physicochemical properties of GBC were characterized by proximate and ultimate analysis, BET, SEM, FTIR, and Raman spectroscopy. The adsorption batch experiment was carried out at various pH values (pH 3–10), mixing times (up to 24 h), and initial CFN concentration (10–500 mg/L). The FTIR analysis revealed the loss of polar functional groups on the surface of GBC derived at high temperatures. The red-shifted and blue-shifted Raman peaks indicate the condensation of small molecules on GBC. The GBC derived at 700 °C demonstrated high CFN adsorption capacity (16.26 mg/g) due to its high surface area and aromaticity. The highest adsorption of CFN was occurred at acidic pH range from 3.5 to 4.5 due to the existence of non-specific attraction between CFN and GBC. The kinetics and isotherm experimental data were fitted with Elovich and fractional power kinetic regression, Freundlich, and Temkin isotherm models, which suggested the adsorption of CFN on the GBC by mixed mechanisms; physisorption and chemisorption including π–π interactions, hydrogen bonding, n–π interactions, electrostatic attraction, and electron donor-acceptor attraction. Moreover, both surface area and aromaticity index have demonstrated a high positive correlation for CFN adsorption, signifying the importance of controlling physicochemical properties based on the end-user purpose of biochar.
中文翻译:

千里胶生物炭去除咖啡因:热解温度和理化特性的影响。
本研究旨在设想理化性质对幽门螺杆菌性能的影响生物炭(GBC)在300、500和700°C下于咖啡因(CFN)中热解;药品和个人护理产品,取自水。GBC的理化特性通过最近和最终分析,BET,SEM,FTIR和拉曼光谱法进行表征。在各种pH值(pH 3-10),混合时间(长达24小时)和CFN初始浓度(10-500 mg / L)下进行吸附批量实验。FTIR分析揭示了高温下GBC表面极性官能团的损失。红移和蓝移的拉曼峰表明小分子在GBC上的凝聚。衍生于700°C的GBC由于具有高的表面积和芳香性,因此具有较高的CFN吸附容量(16.26 mg / g)。CFN的最高吸附发生在3.5至4的酸性pH范围内。由于CFN和GBC之间存在非特定的吸引力,因此使用图5。动力学和等温线实验数据符合Elovich和分数幂动力学回归,Freundlich和Temkin等温线模型,这表明CFN通过混合机制吸附在GBC上。物理吸附和化学吸附,包括π-π相互作用,氢键,n-π相互作用,静电吸引和电子给体-受体吸引。此外,表面积和芳香指数均显示出与CFN吸附的高度正相关性,这表明根据生物炭的最终用户目的控制理化性质的重要性。以及Temkin等温模型,表明CFN通过混合机理在GBC上的吸附;物理吸附和化学吸附,包括π-π相互作用,氢键,n-π相互作用,静电吸引和电子给体-受体吸引。此外,表面积和芳香指数均显示出与CFN吸附的高度正相关性,这表明根据生物炭的最终用户目的控制理化性质的重要性。以及Temkin等温模型,表明CFN通过混合机理在GBC上的吸附;物理吸附和化学吸附,包括π-π相互作用,氢键,n-π相互作用,静电吸引和电子给体-受体吸引。此外,表面积和芳香指数均显示出与CFN吸附的高度正相关性,这表明根据生物炭的最终用户目的控制理化性质的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号