Environmental Pollution ( IF 8.9 ) Pub Date : 2020-07-04 , DOI: 10.1016/j.envpol.2020.115102 Chandi Patra 1 , Rishabh Gupta 1 , Das Bedadeep 1 , Selvaraju Narayanasamy 1
|
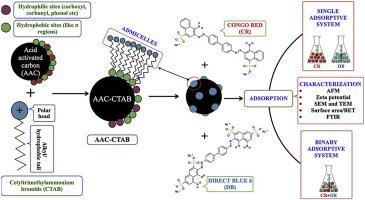
Current study deals with the surface modification of acid activated carbon (prepared from Pongamia pinnata shells) with Cetyltrimethylammonium bromide (CTAB) and its role as an adsorbent in eliminating anionic azo dyes viz. Congo red (CR) and Direct blue 6 (DB) from single and binary adsorptive systems. Binary adsorptive system involved the synergistic and antagonistic influence of one dye over the adsorption of other dye. Physico-chemical alterations due to surfactant modification and post adsorption were studied using atomic force microscopy (AFM), Zeta Potential, scanning electron microscopy (SEM), Energy-dispersive X-ray spectroscopy (EDS), surface area analysis and Fourier-transformed infrared spectroscopy (FTIR). Process parameters influencing efficient adsorption of CR and DB species viz. initial pH of dye solution, adsorbent dosage, incubation temperature and initial concentration of dye species were optimised. Sorbate-sorbent interaction studies for single adsorptive system revealed sorbate's monolayer formation over adsorbent's surface and the involvement of chemisorption, as verified by Langmuir isotherm model and pseudo-second order model, respectively. Langmuir maximum adsorption capacity of the adsorbent was 555.56 mg/g for CR and 625.00 mg/g for DB. Meanwhile, for binary adsorptive system, competitive Langmuir model verified both CR and DB had antagonistic/competitive effect over each other's adsorption. Thermodynamic analysis revealed the adsorptive process as exothermic, spontaneous and thermodynamically favourable with an elevated degree of dis-orderedness. Co-existing cations and anions has nominal effect on the adsorption capacity of dyes. Recyclability studies verified a modest efficiency of 62.52% for CR and 50.47% for DB species after the end of 4th adsorption-desorption cycle; thus affirming its recyclability potential. Phytotoxic assay affirmed the effectivity of the adsorbent in adsorbing dye species from aqueous solutions using Vigna mungo seeds as the model.
中文翻译:

表面处理过的酸活化碳,用于吸附来自单一和二元吸附系统的阴离子偶氮染料:详细的见解。
当前的研究涉及酸活性炭的表面改性(由Pongamia pinnata制备壳)与十六烷基三甲基溴化铵(CTAB)及其作为消除阴离子偶氮染料的吸附剂的作用。来自单一和二元吸附系统的刚果红(CR)和直接蓝6(DB)。二元吸附系统涉及一种染料对另一种染料的吸附的协同和拮抗作用。使用原子力显微镜(AFM),Zeta电位,扫描电子显微镜(SEM),能量色散X射线光谱(EDS),表面积分析和傅里叶变换红外光谱研究了由于表面活性剂改性和后吸附而引起的物理化学变化光谱(FTIR)。影响CR和DB物质有效吸附的工艺参数,即。优化了染料溶液的初始pH,吸附剂用量,温育温度和染料种类的初始浓度。分别通过Langmuir等温模型和伪二级模型验证,对单一吸附系统的吸附剂-吸附剂相互作用研究表明,吸附剂在吸附剂表面形成单层,并参与化学吸附。Langmuir吸附剂的最大吸附容量对CR为555.56 mg / g,对于DB为625.00 mg / g。同时,对于二元吸附系统,竞争Langmuir模型验证了CR和DB对彼此的吸附都具有拮抗/竞争作用。热力学分析表明,吸附过程是放热的,自发的和热力学上有利的,且无序程度增加。共存的阳离子和阴离子对染料的吸附能力有名义上的影响。可回收性研究证明CR和50的适度效率为62.52%。在第四次吸附-解吸循环结束后,DB物种的比例为47%;因此肯定了其可回收性。植物毒性测定法证实了吸附剂在吸附水溶液中从染料中吸收染料的有效性。Vigna mungo种子作为模型。



























 京公网安备 11010802027423号
京公网安备 11010802027423号