当前位置:
X-MOL 学术
›
Chem. Phys. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electronic structure and bonding of the ScNH and YNH molecules
Chemical Physics Letters ( IF 2.8 ) Pub Date : 2020-07-04 , DOI: 10.1016/j.cplett.2020.137735 Soumen Bhattacharyya , James F. Harrison
中文翻译:

ScNH和YNH分子的电子结构和键合
更新日期:2020-07-14
Chemical Physics Letters ( IF 2.8 ) Pub Date : 2020-07-04 , DOI: 10.1016/j.cplett.2020.137735 Soumen Bhattacharyya , James F. Harrison
|
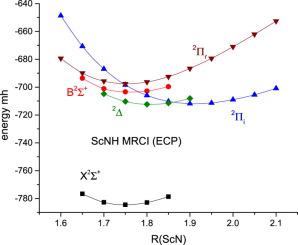
We report the results of multi-reference configuration interaction calculations of the electronic and geometric structures of the 2Σ+, 2Σ+, Ã2Πi, Ã2Πr & 2Δ states of the transition metal imides, ScNH and YNH, and compare with the available experimental data. The metal is bonded to the NH group by a double bond in the system for all states. There are 4 electrons in the 2Σ+, 2Σ+, Ã2Πr & 2Δ states and 3 in the Ã2Πi. The atomic orbital composition in the 4 states is remarkably similar, the average being while in the 3 state we have .
中文翻译:

ScNH和YNH分子的电子结构和键合
我们报告了电子和几何结构的多参考配置相互作用计算的结果 2 Σ +,2 Σ +,A 2 Π我,A 2 Π - [R &2 Δ的过渡金属酰亚胺,ScNH和YNH的状态,并与现有的实验数据进行比较。金属通过双键在NH基团上键合所有州的系统。有4个 电子中的 2 Σ +,2 Σ +,A 2 Π - [R &2 Δ状态和3在A 2 Π我。4中的原子轨道组成 状态非常相似,平均 而在3 说我们有 。



























 京公网安备 11010802027423号
京公网安备 11010802027423号