Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-07-04 , DOI: 10.1016/j.bmc.2020.115608 Buthina A Al-Oudat 1 , Hana'a M Jaradat 1 , Qosay A Al-Balas 1 , Nizar A Al-Shar'i 1 , Amanda Bryant-Friedrich 2 , Mel F Bedi 2
|
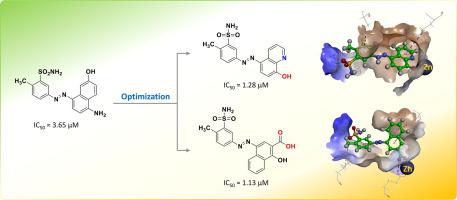
The enzyme glyoxalase-I (Glo-I) is an essential therapeutic target in cancer treatment. Significant efforts have been made to discover competitive inhibitors of Glo-I as potential anticancer agents. Herein, we report the synthesis of a series of diazenylbenzenesulfonamide derivatives, their in vitro evaluation against Glo-I and the resulting structure–activity relationships. Among the compounds tested, compounds 9h and 9j exhibited the highest activity with IC50 1.28 µM and 1.13 µM, respectively. Docking studies to explore the binding mode of the compounds identified key moieties that may contribute to the observed activities. The active compounds will serve as suitable leads for further chemical optimization.
中文翻译:

具有二氮烯基苯磺酰胺部分的新型乙二醛酶 I 抑制剂作为潜在抗癌药物的设计、合成和生物学评价。
乙二醛酶-I (Glo-I) 是癌症治疗中的重要治疗靶点。为了发现作为潜在抗癌药物的 Glo-I 竞争性抑制剂,人们付出了巨大的努力。在此,我们报告了一系列二氮烯基苯磺酰胺衍生物的合成、它们针对 Glo-I 的体外评价以及由此产生的构效关系。在测试的化合物中,化合物9h和9j表现出最高的活性,IC 50分别为1.28 µM和1.13 µM。探索化合物结合模式的对接研究确定了可能有助于观察到的活性的关键部分。活性化合物将作为进一步化学优化的合适先导化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号