Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 2.8 ) Pub Date : 2020-07-04 , DOI: 10.1016/j.bbagen.2020.129680 Naimat K Bari 1 , Jagadish P Hazra 2 , Gaurav Kumar 1 , Simerpreet Kaur 1 , Sharmistha Sinha 1
|
Background
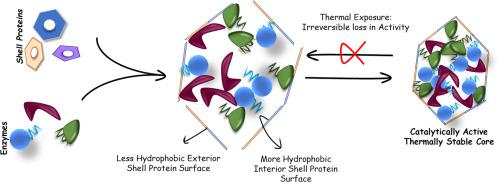
Bacterial microcompartments represent the only reported category of prokaryotic organelles that are capable of functioning as independent bioreactors. In this organelle, a biochemical pathway with all the enzyme machinery is encapsulated within an all protein shell. The shell proteins and the enzymes have distinct structural features. It is hypothesized that flat shell proteins align sideways to form extended sheets and, the globular enzymes fill up the central core of the organelle.
Methods
Using differential scanning fluorimetry, we explored the structure and functional alteration of Pdu BMC, involving tertiary or quaternary structures.
Results
Our findings exhibit that these intact BMCs as a whole behave similar to a globular protein with a rich hydrophobic core, which is exposed upon thermal insult. The encapsulated enzymes itself have a strong hydrophobic core, which is in line with the hydrophobic-collapse model of protein folding. The shell proteins, on the other hand, do not have a strong hydrophobic core and show a significant portion of exposed hydrophobic patches.
Conclusion
We show for the first time the thermal unfolding profile of the BMC domain proteins and the unique exposure of hydrophobic patches in them might be required for anchoring the enzymes leading to better packaging of the micro-compartments.
General significance
These observations indicate that the genesis of these unique bacterial organelles is driven by the hydrophobic interactions between the shell and the enzymes. Insights from this work will aid in the genetic and biochemical engineering of thermostable efficient enzymatic biomaterials.
中文翻译:

使用热扫描法探测多蛋白原核细胞器揭示了核和壳的独特特性。
背景
细菌微区室是唯一报道的能够作为独立生物反应器起作用的原核细胞器的类别。在该细胞器中,具有所有酶机制的生化途径被封装在全蛋白壳内。壳蛋白和酶具有独特的结构特征。据推测,扁平壳蛋白侧向排列以形成延伸的片,并且球状酶填充了细胞器的中央核心。
方法
使用差示扫描荧光法,我们探讨了Pdu BMC的结构和功能变化,涉及三级或四级结构。
结果
我们的发现表明,这些完整的BMC整体上与具有丰富疏水核心的球状蛋白质相似,该蛋白质在受热时会暴露出来。封装的酶本身具有强大的疏水性核心,这与蛋白质折叠的疏水性折叠模型一致。另一方面,壳蛋白不具有强的疏水性核心,并且显示出暴露的疏水性斑块的显着部分。
结论
我们第一次显示出BMC结构域蛋白的热展开图谱和疏水性斑块在其中的独特暴露可能是锚定酶所需的,从而导致微区室的更好包装。
一般意义
这些观察结果表明,这些独特的细菌细胞器的发生是由壳与酶之间的疏水相互作用驱动的。这项工作的见识将有助于热稳定高效酶生物材料的遗传和生物化学工程。









































 京公网安备 11010802027423号
京公网安备 11010802027423号