当前位置:
X-MOL 学术
›
Anal. Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gold nanoparticle-engineered electrochemical aptamer biosensor for ultrasensitive detection of thrombin.
Analytical Methods ( IF 2.7 ) Pub Date : 2020-07-03 , DOI: 10.1039/d0ay01163k Ying Chen 1 , Junyi Xiang , Bin Liu , Zhengbo Chen , Xia Zuo
Analytical Methods ( IF 2.7 ) Pub Date : 2020-07-03 , DOI: 10.1039/d0ay01163k Ying Chen 1 , Junyi Xiang , Bin Liu , Zhengbo Chen , Xia Zuo
Affiliation
|
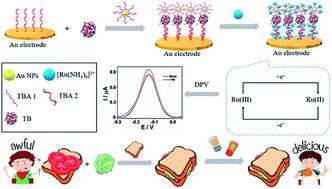
In order to obtain a lower detection limit in electrochemical detection, the choice of signal amplification strategy is of great importance. In this work, we describe an electrochemical sandwich aptasensor based on a signal amplification system involving two thrombin (TB) aptamers (TBA1 and TBA2), gold nanoparticles (AuNPs) as aptamer carriers, and [Ru(NH3)6]3+ for signal conversion. In the presence of the target thrombin, TBA1 and TBA2 specifically bind to TB, and the TBA1–TB–TBA2 complexes cause the formation of a sandwich structure, meaning more [Ru(NH3)6]3+ can be adsorbed on the negatively charged phosphate backbone of the aptamers, resulting in an increase in the differential pulse voltammetry (DPV) current. Under optimal conditions, the aptasensor exhibited a linear range of 1 fM to 6 pM and a limit of detection of 0.1429 fM (S/N = 3) for TB. The proposed aptasensor displayed an excellent selectivity and reproducibility. Importantly, the aptasensor was capable of detecting TB in serum samples successfully.
中文翻译:

金纳米粒子工程电化学适体生物传感器,用于凝血酶的超灵敏检测。
为了在电化学检测中获得较低的检测限,信号放大策略的选择非常重要。在这项工作中,我们描述了基于涉及两个凝血酶(TB)的适体(TBA1和TBA2),金粒子(AuNPs)作为适体的载体,和的[Ru(NH一个信号放大系统上的电化学夹层适体传感器3)6 ] 3+为信号转换。在存在目标凝血酶的情况下,TBA1和TBA2与TB特异性结合,TBA1-TB-TBA2复合物会形成夹心结构,这意味着[Ru(NH 3)6 ] 3+分子被吸附在适体的带负电荷的磷酸骨架上,导致差分脉冲伏安法(DPV)电流增加。在最佳条件下,适体传感器的线性范围为1 fM至6 pM,对TB的检出限为0.1429 fM(S / N = 3)。拟议的适体传感器显示出极好的选择性和可重复性。重要的是,aptasensor能够成功检测血清样品中的TB。
更新日期:2020-07-30
中文翻译:

金纳米粒子工程电化学适体生物传感器,用于凝血酶的超灵敏检测。
为了在电化学检测中获得较低的检测限,信号放大策略的选择非常重要。在这项工作中,我们描述了基于涉及两个凝血酶(TB)的适体(TBA1和TBA2),金粒子(AuNPs)作为适体的载体,和的[Ru(NH一个信号放大系统上的电化学夹层适体传感器3)6 ] 3+为信号转换。在存在目标凝血酶的情况下,TBA1和TBA2与TB特异性结合,TBA1-TB-TBA2复合物会形成夹心结构,这意味着[Ru(NH 3)6 ] 3+分子被吸附在适体的带负电荷的磷酸骨架上,导致差分脉冲伏安法(DPV)电流增加。在最佳条件下,适体传感器的线性范围为1 fM至6 pM,对TB的检出限为0.1429 fM(S / N = 3)。拟议的适体传感器显示出极好的选择性和可重复性。重要的是,aptasensor能够成功检测血清样品中的TB。











































 京公网安备 11010802027423号
京公网安备 11010802027423号