当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Convergent Enantioselective Synthetic Route to (-)-Myrocin G.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-07-02 , DOI: 10.1021/acs.joc.0c00891 Martin Tomanik 1 , Christos Economou 1 , Madeline C Frischling 1 , Mengzhao Xue 1 , Victoria A Marks 1 , Brandon Q Mercado 1 , Seth B Herzon 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-07-02 , DOI: 10.1021/acs.joc.0c00891 Martin Tomanik 1 , Christos Economou 1 , Madeline C Frischling 1 , Mengzhao Xue 1 , Victoria A Marks 1 , Brandon Q Mercado 1 , Seth B Herzon 1, 2
Affiliation
|
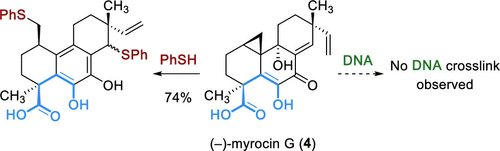
Myrocins are a family of antiproliferative antibiotic fungal metabolites possessing a masked electrophilic cyclopropane. Preliminary chemical reactivity studies imputed the bioactivity of these natural products to a DNA cross-linking mechanism, but this hypothesis was not confirmed by studies with native DNA. We recently reported a total synthesis of (−)-myrocin G (4), the putative active form of the metabolite myrocin C (1), that featured a carefully orchestrated tandem fragment coupling–annulation cascade. Herein, we describe the evolution of our synthetic strategy toward 4 and report the series of discoveries that prompted the design of this cascade coupling. Efforts to convert the diosphenol (−)-myrocin G (4) to the corresponding 5-hydroxy-γ-lactone isomer myrocin C (1) are also detailed. We present a preliminary evaluation of the antiproliferative activities of (−)-myrocin G (4) and related structures, as well as DNA cross-linking studies. These studies indicate that myrocins do not cross-link DNA, suggesting an alternative mode of action potentially involving a protein target.
中文翻译:

(-)-Myrocin G的收敛对映选择性合成路线的发展。
Myrocins是具有掩蔽的亲电环丙烷的抗增殖抗生素真菌代谢产物家族。初步的化学反应性研究将这些天然产物的生物活性归因于DNA交联机制,但对天然DNA的研究并未证实这一假设。我们最近报道了代谢产物myrocin C(1)的推定活性形式(-)-myrocin G(4)的总合成,其特征是精心设计的串联片段偶联-环流级联反应。在此,我们描述了合成策略向4的演变,并报告了促使该级联耦合设计的一系列发现。努力转化二酚(-)-myrocin G(4)到相应的5-羟基-γ-内酯异构体myrocin C(1)也有详述。我们目前对(-)-myrocin G(4)和相关结构的抗增殖活性进行初步评估,以及DNA交联研究。这些研究表明,myrocins不会使DNA交联,表明可能涉及蛋白质靶标的另一种作用方式。
更新日期:2020-07-17
中文翻译:

(-)-Myrocin G的收敛对映选择性合成路线的发展。
Myrocins是具有掩蔽的亲电环丙烷的抗增殖抗生素真菌代谢产物家族。初步的化学反应性研究将这些天然产物的生物活性归因于DNA交联机制,但对天然DNA的研究并未证实这一假设。我们最近报道了代谢产物myrocin C(1)的推定活性形式(-)-myrocin G(4)的总合成,其特征是精心设计的串联片段偶联-环流级联反应。在此,我们描述了合成策略向4的演变,并报告了促使该级联耦合设计的一系列发现。努力转化二酚(-)-myrocin G(4)到相应的5-羟基-γ-内酯异构体myrocin C(1)也有详述。我们目前对(-)-myrocin G(4)和相关结构的抗增殖活性进行初步评估,以及DNA交联研究。这些研究表明,myrocins不会使DNA交联,表明可能涉及蛋白质靶标的另一种作用方式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号