当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Indolizines Enabling Rapid Uncaging of Alcohols and Carboxylic Acids by Red Light-Induced Photooxidation.
Organic Letters ( IF 4.9 ) Pub Date : 2020-07-03 , DOI: 10.1021/acs.orglett.0c01799 Kenji Watanabe 1 , Nodoka Terao 1 , Isao Kii 1, 2, 3 , Reiko Nakagawa 4 , Takashi Niwa 1 , Takamitsu Hosoya 1, 5
Organic Letters ( IF 4.9 ) Pub Date : 2020-07-03 , DOI: 10.1021/acs.orglett.0c01799 Kenji Watanabe 1 , Nodoka Terao 1 , Isao Kii 1, 2, 3 , Reiko Nakagawa 4 , Takashi Niwa 1 , Takamitsu Hosoya 1, 5
Affiliation
|
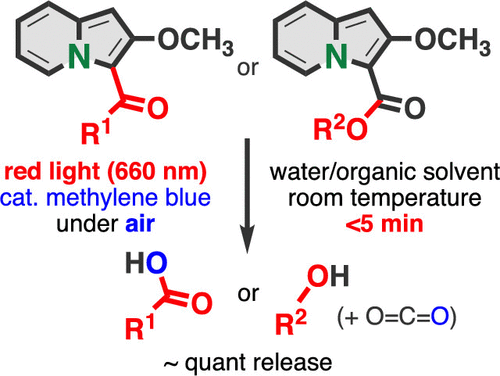
The irradiation of red light-emitting-diode light (λ = 660 nm) to 3-acyl-2-methoxyindolizines in the presence of a catalytic amount of methylene blue triggered the photooxidation of the indolizine ring, resulting in a nearly quantitative release of alcohols or carboxylic acids within a few minutes. The method was applicable for photouncaging various functional molecules such as a carboxylic anticancer drug and a phenolic fluorescent dye from the corresponding indolizine conjugates, including an insulin–indolizine–dye conjugate.
中文翻译:

吲哚嗪使红光诱导的光氧化作用迅速释放出醇和羧酸。
在催化量的亚甲基蓝的存在下,将红色发光二极管光(λ= 660 nm)照射到3-酰基-2-甲氧基吲哚并引发吲哚嗪环的光氧化,从而导致几乎定量地释放出醇类或在几分钟内用羧酸。该方法适用于从相应的吲哚利嗪缀合物(包括胰岛素-吲哚嗪-染料缀合物)中对各种功能分子(例如羧酸抗癌药和酚类荧光染料)进行光解开。
更新日期:2020-07-17
中文翻译:

吲哚嗪使红光诱导的光氧化作用迅速释放出醇和羧酸。
在催化量的亚甲基蓝的存在下,将红色发光二极管光(λ= 660 nm)照射到3-酰基-2-甲氧基吲哚并引发吲哚嗪环的光氧化,从而导致几乎定量地释放出醇类或在几分钟内用羧酸。该方法适用于从相应的吲哚利嗪缀合物(包括胰岛素-吲哚嗪-染料缀合物)中对各种功能分子(例如羧酸抗癌药和酚类荧光染料)进行光解开。











































 京公网安备 11010802027423号
京公网安备 11010802027423号