当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Multifunctional Platform for the Capture, Release, And Enumeration of Circulating Tumor Cells Based on Aptamer Binding, Nicking Endonuclease-Assisted Amplification, And Inductively Coupled Plasma Mass Spectrometry Detection.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-07-02 , DOI: 10.1021/acs.analchem.0c00276 Xiao Yin 1 , Beibei Chen 1 , Man He 1 , Bin Hu 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-07-02 , DOI: 10.1021/acs.analchem.0c00276 Xiao Yin 1 , Beibei Chen 1 , Man He 1 , Bin Hu 1
Affiliation
|
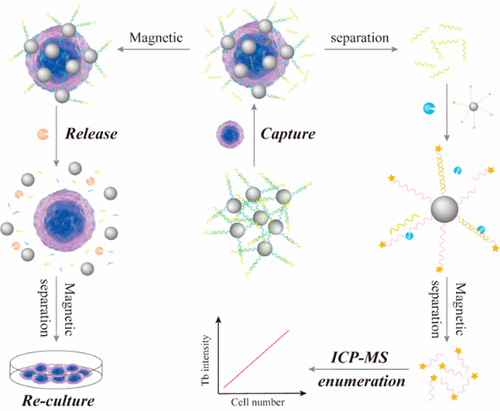
Inductively coupled plasma mass spectrometry (ICP-MS) combined with element tags has been well designed and extensively applied in cell enumeration. It possesses superior quantitative capability, strong resistance to matrix interference, multiplex detection capability but destructive characteristic. Herein, we constructed an ICP-MS based multifunctional platform for capture, nondestructive enumeration, and release of circulating tumor cells (CTCs). Aptamer on capture probe recognized Mucin 1 (MUC1) on membrane of MCF-7 cells specifically, thus the cells were captured by probe and the Initiator originally hybridized with Aptamer was substituted by MUC1 and released into solution. Then the released Initiator was separated from the captured cells and hybridized with Tb labeled Substrate on detection probe to release a large amount of nicked Tb fragments through the nicking endonuclease assisted amplification for subsequent ICP-MS detection. Meanwhile, cells captured by probe were released by nuclease digestion for further reculture. Such a strategy effectively avoids CTCs destruction resulted from ICP-MS enumeration, increases the detection sensitivity of ICP-MS by involving nicking endonuclease assisted amplification, and realizes cell recovery for further analysis. A limit of detection of 87 MCF-7 cells and a linear range of 250–10 000 MCF-7 cells were realized for ICP-MS enumeration. A cell recovery of 52.7% (with capture and release efficiency of 63.9 and 82.5%, respectively) and a viability of 74.3% were obtained, meanwhile the released cells exhibited strong proliferation ability. This multifunctional platform for CTCs capture, enumeration, and release has great applicable potential in early diagnosis and individual treatment for cancer.
中文翻译:

基于适体结合,核酸内切酶辅助扩增和电感耦合等离子体质谱检测的循环肿瘤细胞捕获,释放和计数的多功能平台。
结合元素标签的电感耦合等离子体质谱法(ICP-MS)已经过精心设计,并广泛应用于细胞计数。具有定量能力强,抗基质干扰能力强,多重检测能力强,破坏性强的特点。本文中,我们构建了基于ICP-MS的多功能平台,用于捕获,无损枚举和释放循环肿瘤细胞(CTC)。捕获探针上的适体特异性识别MCF-7细胞膜上的粘蛋白1(MUC1),因此探针捕获了细胞,最初与Aptamer杂交的引发剂被MUC1取代并释放到溶液中。然后将释放的引发剂与捕获的细胞分离,并与Tb标记的检测探针上的底物杂交,通过带切口的核酸内切酶辅助扩增释放大量带切口的Tb片段,用于后续的ICP-MS检测。同时,被核酸捕获的探针释放出被探针捕获的细胞,以进行进一步的培养。这种策略有效地避免了由ICP-MS枚举引起的CTC破坏,通过涉及切口核酸内切酶辅助扩增而提高了ICP-MS的检测灵敏度,并实现了细胞回收以进行进一步分析。ICP-MS枚举的检测限为87个MCF-7细胞,线性范围为250–10 000 MCF-7细胞。细胞回收率为52.7%(捕获和释放效率分别为63.9和82.5%)和生存力为74.3%,同时释放的细胞具有较强的增殖能力。这个用于CTC捕获,枚举和释放的多功能平台在癌症的早期诊断和个体治疗中具有巨大的应用潜力。
更新日期:2020-08-04
中文翻译:

基于适体结合,核酸内切酶辅助扩增和电感耦合等离子体质谱检测的循环肿瘤细胞捕获,释放和计数的多功能平台。
结合元素标签的电感耦合等离子体质谱法(ICP-MS)已经过精心设计,并广泛应用于细胞计数。具有定量能力强,抗基质干扰能力强,多重检测能力强,破坏性强的特点。本文中,我们构建了基于ICP-MS的多功能平台,用于捕获,无损枚举和释放循环肿瘤细胞(CTC)。捕获探针上的适体特异性识别MCF-7细胞膜上的粘蛋白1(MUC1),因此探针捕获了细胞,最初与Aptamer杂交的引发剂被MUC1取代并释放到溶液中。然后将释放的引发剂与捕获的细胞分离,并与Tb标记的检测探针上的底物杂交,通过带切口的核酸内切酶辅助扩增释放大量带切口的Tb片段,用于后续的ICP-MS检测。同时,被核酸捕获的探针释放出被探针捕获的细胞,以进行进一步的培养。这种策略有效地避免了由ICP-MS枚举引起的CTC破坏,通过涉及切口核酸内切酶辅助扩增而提高了ICP-MS的检测灵敏度,并实现了细胞回收以进行进一步分析。ICP-MS枚举的检测限为87个MCF-7细胞,线性范围为250–10 000 MCF-7细胞。细胞回收率为52.7%(捕获和释放效率分别为63.9和82.5%)和生存力为74.3%,同时释放的细胞具有较强的增殖能力。这个用于CTC捕获,枚举和释放的多功能平台在癌症的早期诊断和个体治疗中具有巨大的应用潜力。









































 京公网安备 11010802027423号
京公网安备 11010802027423号