Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Batch and Continuous Fixed-Bed Lead Removal Using Himalayan Pine Needle Biochar: Isotherm and Kinetic Studies.
ACS Omega ( IF 3.7 ) Pub Date : 2020-07-02 , DOI: 10.1021/acsomega.0c00216 Vaishali Choudhary 1 , Manvendra Patel 1 , Charles U Pittman 2 , Dinesh Mohan 1
ACS Omega ( IF 3.7 ) Pub Date : 2020-07-02 , DOI: 10.1021/acsomega.0c00216 Vaishali Choudhary 1 , Manvendra Patel 1 , Charles U Pittman 2 , Dinesh Mohan 1
Affiliation
|
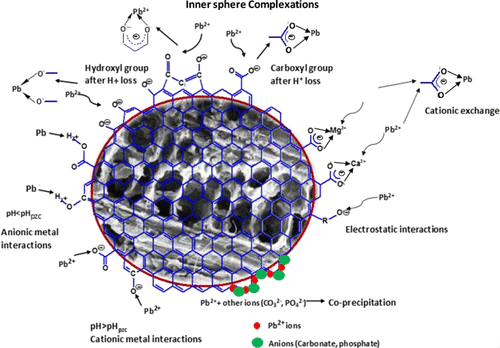
Pine needle litter in Himalayan forests leads to forest fires, ground water recharge inhibition, soil acidification and contamination, and stops the growth of grass and plants. This study provides a possible solution for pine needle litter problem by converting it to biochar. Pine needle litter lying on the ground for approximately a month was collected from the Himalayan region. The pine needle litter biochars were generated using slow pyrolysis (residence time, 30 min; heating rate, 10 °C/min) at 350, 450, 550, 650, and 750 °C. Finally, pine needle litter biochar prepared at 550 °C (PNBC550) was selected for sorptive removal of aqueous lead both in batch and column studies. The PNBC550 was characterized for proximate and elemental compositions, crystallinity, surface area, morphology, and functional groups. A BET surface area of 230.9 m2/g was obtained for PNBC550. Batch sorption studies were carried out to study (1) the adsorption versus pH studies (at pH 2 to 7), (2) isotherms (at 10, 25, and 35 °C) to evaluate the temperature effect on the sorption efficiency, and (3) kinetics to reveal the effect of time, adsorbent dose, and initial concentration on the reaction rate. Increasing pyrolysis temperature raised lead sorption up to 550 °C. Lead adsorption increased considerably as pH rose from 2 to a maximum adsorption around pH 5 and above. The sorption data were fitted using different isotherm models and kinetic equations. The Langmuir adsorption capacity increased from 22.93 mg/g at 10 °C to 40.43 mg/g at 35 °C, showing that adsorption was endothermic. Fixed-bed studies were conducted at room temperature with an initial lead concentration of 7.85 mg/L and 4.0 g of PNBC550 at initial pH 5.0 and a flow rate of 3 mL/min. Desorption studies conducted under the same experimental conditions found about 90–93% lead recovery. Development of high-efficiency biochars for lead remediation provides a sustainable solution for the Himalayan pine needle litter problem. The biochars also possess the possible potential for aqueous removal of other metal cations.
中文翻译:

使用喜马拉雅松针生物炭批量和连续固定床除铅:等温线和动力学研究。
喜马拉雅森林中的松针凋落物会导致森林火灾、抑制地下水补给、土壤酸化和污染,并阻止草和植物的生长。这项研究通过将松针垃圾转化为生物炭,为解决松针垃圾问题提供了可能的解决方案。从喜马拉雅地区收集了地上大约一个月的松针垃圾。松针凋落物生物炭是通过在 350、450、550、650 和 750 °C 下缓慢热解(停留时间,30 分钟;加热速率,10 °C/分钟)生成的。最后,选择在 550 °C (PNBC550) 下制备的松针凋落物生物炭,用于在批量和柱研究中吸附去除水性铅。 PNBC550 的特征包括近似成分和元素成分、结晶度、表面积、形态和官能团。 PNBC550的BET表面积为230.9m 2 /g。进行批量吸附研究是为了研究 (1) 吸附与 pH 的关系研究(pH 2 至 7),(2) 等温线(10、25 和 35 °C)以评估温度对吸附效率的影响,以及(3) 动力学揭示时间、吸附剂剂量和初始浓度对反应速率的影响。增加热解温度将铅吸附提高至 550 °C。当 pH 值从 2 升至 5 左右及以上时,铅的吸附量显着增加。使用不同的等温线模型和动力学方程拟合吸附数据。 Langmuir吸附容量从10℃时的22.93mg/g增加到35℃时的40.43mg/g,表明吸附是吸热的。固定床研究在室温下进行,初始铅浓度为 7.85 mg/L,初始 pH 为 5.0,流速为 3 mL/min,PNBC550 为 4.0 g。 在相同实验条件下进行的解吸研究发现铅回收率约为 90-93%。开发用于铅修复的高效生物炭为喜马拉雅松针凋落物问题提供了可持续的解决方案。生物炭还具有去除水中其他金属阳离子的可能潜力。
更新日期:2020-07-14
中文翻译:

使用喜马拉雅松针生物炭批量和连续固定床除铅:等温线和动力学研究。
喜马拉雅森林中的松针凋落物会导致森林火灾、抑制地下水补给、土壤酸化和污染,并阻止草和植物的生长。这项研究通过将松针垃圾转化为生物炭,为解决松针垃圾问题提供了可能的解决方案。从喜马拉雅地区收集了地上大约一个月的松针垃圾。松针凋落物生物炭是通过在 350、450、550、650 和 750 °C 下缓慢热解(停留时间,30 分钟;加热速率,10 °C/分钟)生成的。最后,选择在 550 °C (PNBC550) 下制备的松针凋落物生物炭,用于在批量和柱研究中吸附去除水性铅。 PNBC550 的特征包括近似成分和元素成分、结晶度、表面积、形态和官能团。 PNBC550的BET表面积为230.9m 2 /g。进行批量吸附研究是为了研究 (1) 吸附与 pH 的关系研究(pH 2 至 7),(2) 等温线(10、25 和 35 °C)以评估温度对吸附效率的影响,以及(3) 动力学揭示时间、吸附剂剂量和初始浓度对反应速率的影响。增加热解温度将铅吸附提高至 550 °C。当 pH 值从 2 升至 5 左右及以上时,铅的吸附量显着增加。使用不同的等温线模型和动力学方程拟合吸附数据。 Langmuir吸附容量从10℃时的22.93mg/g增加到35℃时的40.43mg/g,表明吸附是吸热的。固定床研究在室温下进行,初始铅浓度为 7.85 mg/L,初始 pH 为 5.0,流速为 3 mL/min,PNBC550 为 4.0 g。 在相同实验条件下进行的解吸研究发现铅回收率约为 90-93%。开发用于铅修复的高效生物炭为喜马拉雅松针凋落物问题提供了可持续的解决方案。生物炭还具有去除水中其他金属阳离子的可能潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号