当前位置:
X-MOL 学术
›
Photochem. Photobiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fatty Acid Conjugates of Toluidine Blue O as Amphiphilic Photosensitizers: Synthesis, Solubility, Photophysics, and Photochemical Properties
Photochemistry and Photobiology ( IF 2.6 ) Pub Date : 2020-07-23 , DOI: 10.1111/php.13304 José Robinson-Duggon 1, 2 , Nancy Pizarro 3 , Germán Gunther 4 , Daniel Zúñiga-Núñez 1 , Ana María Edwards 1 , Alexander Greer 5, 6 , Denis Fuentealba 1
Photochemistry and Photobiology ( IF 2.6 ) Pub Date : 2020-07-23 , DOI: 10.1111/php.13304 José Robinson-Duggon 1, 2 , Nancy Pizarro 3 , Germán Gunther 4 , Daniel Zúñiga-Núñez 1 , Ana María Edwards 1 , Alexander Greer 5, 6 , Denis Fuentealba 1
Affiliation
|
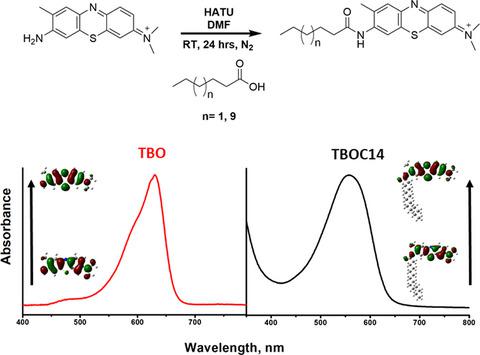
Toluidine blue O (TBO) is a water‐soluble photosensitizer that has been used in photodynamic antimicrobial and anticancer treatments, but suffers from limited solubility in hydrophobic media. In an effort to incrementally increase TBO’s hydrophobicity, we describe the synthesis of hexanoic (TBOC6) and myristic (TBOC14) fatty acid derivatives of TBO formed in low to moderate percent yields by condensation with the free amine site. Covalently linking 6 and 14 carbon chains led to modifications of not only TBO’s solubility, but also its photophysical and photochemical properties. TBOC6 and TBOC14 derivatives were more soluble in organic solvents and showed hypsochromic shifts in their absorption and emission bands. The solubility in phosphate buffer solution was low for both TBOC6 and TBOC14, but unexpectedly slightly greater in the latter. Both TBOC6 and TBOC14 showed decreased triplet excited‐state lifetimes and singlet oxygen quantum yields in acetonitrile, which was attributed to heightened aggregation of these conjugates particularly at high concentrations due to the hydrophobic “tails.” While in diluted aqueous buffer solution, indirect measurements showed similar efficiency in singlet oxygen generation for TBOC14 compared to TBO. This work demonstrates a facile synthesis of fatty acid TBO derivatives leading to amphiphilic compounds with a delocalized cationic “head” group and hydrophobic “tails” for potential to accumulate into biological membranes or membrane/aqueous interfaces in PDT applications.
中文翻译:

作为两亲光敏剂的甲苯胺蓝 O 的脂肪酸缀合物:合成、溶解度、光物理和光化学性质
甲苯胺蓝 O (TBO) 是一种水溶性光敏剂,已用于光动力抗菌和抗癌治疗,但在疏水介质中的溶解度有限。为了逐步增加 TBO 的疏水性,我们描述了通过与游离胺位点缩合形成的 TBO 的己酸 (TBOC6) 和肉豆蔻 (TBOC14) 脂肪酸衍生物的合成,产率从低到中等。共价连接 6 和 14 个碳链不仅会改变 TBO 的溶解度,还会改变其光物理和光化学性质。TBOC6 和 TBOC14 衍生物更易溶于有机溶剂,并在其吸收和发射带中显示出低色移。TBOC6 和 TBOC14 在磷酸盐缓冲液中的溶解度都很低,但出乎意料的是后者略高。TBOC6 和 TBOC14 在乙腈中的三线态激发态寿命和单线态氧量子产率均降低,这归因于这些共轭物的聚集增强,尤其是在高浓度下,由于疏水性“尾部”。而在稀释的缓冲水溶液中,间接测量显示与 TBO 相比,TBOC14 的单线态氧生成效率相似。这项工作证明了脂肪酸 TBO 衍生物的轻松合成,导致两亲化合物具有离域阳离子“头部”基团和疏水“尾部”,有可能在 PDT 应用中积累到生物膜或膜/水界面。这归因于这些缀合物的高度聚集,特别是在高浓度下,由于疏水“尾巴”。而在稀释的缓冲水溶液中,间接测量显示与 TBO 相比,TBOC14 的单线态氧产生效率相似。这项工作证明了脂肪酸 TBO 衍生物的轻松合成,导致两亲化合物具有离域阳离子“头部”基团和疏水“尾部”,有可能在 PDT 应用中积累到生物膜或膜/水界面。这归因于这些缀合物的高度聚集,特别是在高浓度下,由于疏水“尾巴”。而在稀释的缓冲水溶液中,间接测量显示与 TBO 相比,TBOC14 的单线态氧产生效率相似。这项工作证明了脂肪酸 TBO 衍生物的轻松合成,导致两亲化合物具有离域阳离子“头部”基团和疏水“尾部”,有可能在 PDT 应用中积累到生物膜或膜/水界面。
更新日期:2020-07-23
中文翻译:

作为两亲光敏剂的甲苯胺蓝 O 的脂肪酸缀合物:合成、溶解度、光物理和光化学性质
甲苯胺蓝 O (TBO) 是一种水溶性光敏剂,已用于光动力抗菌和抗癌治疗,但在疏水介质中的溶解度有限。为了逐步增加 TBO 的疏水性,我们描述了通过与游离胺位点缩合形成的 TBO 的己酸 (TBOC6) 和肉豆蔻 (TBOC14) 脂肪酸衍生物的合成,产率从低到中等。共价连接 6 和 14 个碳链不仅会改变 TBO 的溶解度,还会改变其光物理和光化学性质。TBOC6 和 TBOC14 衍生物更易溶于有机溶剂,并在其吸收和发射带中显示出低色移。TBOC6 和 TBOC14 在磷酸盐缓冲液中的溶解度都很低,但出乎意料的是后者略高。TBOC6 和 TBOC14 在乙腈中的三线态激发态寿命和单线态氧量子产率均降低,这归因于这些共轭物的聚集增强,尤其是在高浓度下,由于疏水性“尾部”。而在稀释的缓冲水溶液中,间接测量显示与 TBO 相比,TBOC14 的单线态氧生成效率相似。这项工作证明了脂肪酸 TBO 衍生物的轻松合成,导致两亲化合物具有离域阳离子“头部”基团和疏水“尾部”,有可能在 PDT 应用中积累到生物膜或膜/水界面。这归因于这些缀合物的高度聚集,特别是在高浓度下,由于疏水“尾巴”。而在稀释的缓冲水溶液中,间接测量显示与 TBO 相比,TBOC14 的单线态氧产生效率相似。这项工作证明了脂肪酸 TBO 衍生物的轻松合成,导致两亲化合物具有离域阳离子“头部”基团和疏水“尾部”,有可能在 PDT 应用中积累到生物膜或膜/水界面。这归因于这些缀合物的高度聚集,特别是在高浓度下,由于疏水“尾巴”。而在稀释的缓冲水溶液中,间接测量显示与 TBO 相比,TBOC14 的单线态氧产生效率相似。这项工作证明了脂肪酸 TBO 衍生物的轻松合成,导致两亲化合物具有离域阳离子“头部”基团和疏水“尾部”,有可能在 PDT 应用中积累到生物膜或膜/水界面。











































 京公网安备 11010802027423号
京公网安备 11010802027423号