当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Syntheses of Benzo[b]carbazoles and C3‐Substituted Indoles via Tunable Catalytic Annulations of β‐Alkynyl Ketones with Indoles
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-07-02 , DOI: 10.1002/adsc.202000491 Dan Wang 1 , Shi‐Chao Wang 1 , Wen‐Juan Hao 1 , Shu‐Jiang Tu 1 , Bo Jiang 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-07-02 , DOI: 10.1002/adsc.202000491 Dan Wang 1 , Shi‐Chao Wang 1 , Wen‐Juan Hao 1 , Shu‐Jiang Tu 1 , Bo Jiang 1
Affiliation
|
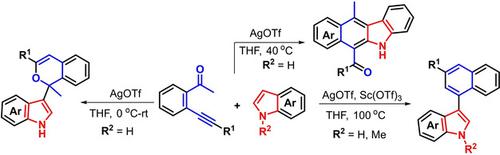
Tunable catalytic annulation reactions of β alkynyl ketones with indoles have been developed, enabling multiple chemical bond‐forming events to selectively access skeletally diverse indole‐containing heterocycles with generally good yields. Silver‐catalyzed intermolecular benzannulation reaction of β‐alkynyl ketones with indoles afforded tetracyclic benzo[b]carbazoles whereas isochromen‐1‐yl‐substituted indoles could be obtained using the same silver catalysis by lowering the reaction temperature (0 °C or rt). Interestingly, using Sc(OTf)3 and AgOTf as a combined catalytic system led to the formation of C3‐naphthylated indoles via intramolecular benzannulation reaction.
中文翻译:

β-炔基酮与吲哚的可调催化环状选择性合成苯并[b]咔唑和C3取代的吲哚
已经开发出可调谐的β炔基酮与吲哚的催化环化反应,使多个化学键形成事件能够以通常良好的收率选择性地进入具有骨架的各种含吲哚杂环。银催化的β-炔基酮与吲哚的分子间苯环化反应可得到四环苯并[ b ]咔唑,而在相同的银催化下,通过降低反应温度(0°C或室温)可以得到异铬烯-1-基取代的吲哚。有趣的是,使用Sc(OTf)3和AgOTf作为联合催化体系,可通过分子内苯环化反应形成C3-萘甲酰吲哚。
更新日期:2020-08-19
中文翻译:

β-炔基酮与吲哚的可调催化环状选择性合成苯并[b]咔唑和C3取代的吲哚
已经开发出可调谐的β炔基酮与吲哚的催化环化反应,使多个化学键形成事件能够以通常良好的收率选择性地进入具有骨架的各种含吲哚杂环。银催化的β-炔基酮与吲哚的分子间苯环化反应可得到四环苯并[ b ]咔唑,而在相同的银催化下,通过降低反应温度(0°C或室温)可以得到异铬烯-1-基取代的吲哚。有趣的是,使用Sc(OTf)3和AgOTf作为联合催化体系,可通过分子内苯环化反应形成C3-萘甲酰吲哚。











































 京公网安备 11010802027423号
京公网安备 11010802027423号