当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The affinity of some Lewis bases for Hexafluoroisopropanol as a reference Lewis acid: an ITC/DFT study.
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-07-03 , DOI: 10.1002/cphc.202000560 Milan R Milovanović 1, 2 , Quentin Dherbassy 3 , Joanna Wencel-Delord 3 , Françoise Colobert 3 , Snežana D Zarić 4 , Jean-Pierre Djukic 2
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-07-03 , DOI: 10.1002/cphc.202000560 Milan R Milovanović 1, 2 , Quentin Dherbassy 3 , Joanna Wencel-Delord 3 , Françoise Colobert 3 , Snežana D Zarić 4 , Jean-Pierre Djukic 2
Affiliation
|
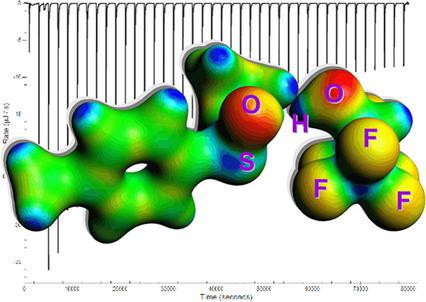
To figure out the possible role of 1,1,1,3,3,3‐hexafluoropropan‐2‐ol (HFIP) as well as to provide reference thermochemical data in solution, the formation of Lewis acid‐base complexes between HFIP (Lewis acid) and a series of 8 different Lewis bases (3 sulfoxides, 3 Nsp2 pyridine derivatives, 1 aromatic amine, 1 cyclic aliphatic ether) was examined by isothermal titration calorimetry (ITC) experiments and static density functional theory augmented with Dispersion (DFT−D) calculations. Measured ITC association enthalpy values (ΔHa) lie between −9.3 and −14 kcal mol−1. Computations including a PCM implicit solvation model produced similar exothermicity of association of all studied systems compared to the ITC data with ΔHa values ranging from −8.5 to −12.7 kcal mol−1. An additional set of calculations combining implicit and explicit solvation by chlorobenzene of the reactants, pointed out the relatively low interference of the solvent with the HFIP‐base complexation: its main effect is to slightly enhance the Gibbs energy of the HFIP‐Lewis base association. It is speculated that the interactions of bulk HFIP with Lewis bases therefore may significantly intervene in catalytic processes not only via the dynamic microstructuring of the medium but also more explicitly by affecting bonds’ polarization at the Lewis bases.
中文翻译:

一些Lewis碱对六氟异丙醇作为参考Lewis酸的亲和力:一项ITC / DFT研究。
为了弄清1,1,1,3,3,3-六氟丙烷-2-醇(HFIP)的可能作用并提供溶液中的参考热化学数据,HFIP之间形成路易斯酸碱配合物(Lewis酸)和一系列8种不同的Lewis碱(3种亚砜,3种Nsp 2吡啶衍生物,1种芳族胺,1种环状脂族醚)通过等温滴定热(ITC)实验和通过分散(DFT- D)计算。测得的ITC缔合焓值(ΔH a)在-9.3和-14 kcal mol -1之间。与具有ΔH a的ITC数据相比,包括PCM隐式溶剂化模型在内的计算都对所有研究系统产生相似的放热关系。值介于-8.5至-12.7 kcal mol -1之间。一组额外的计算结合了反应物的氯苯的隐式和显式溶剂化,指出溶剂对HFIP-碱络合物的干扰相对较低:其主要作用是略微提高HFIP-刘易斯碱缔合的吉布斯能。据推测,本体HFIP与路易斯碱的相互作用因此不仅可以通过介质的动态微结构而且还可以通过影响路易斯碱处的键的极化而更明显地介入催化过程。
更新日期:2020-07-03
中文翻译:

一些Lewis碱对六氟异丙醇作为参考Lewis酸的亲和力:一项ITC / DFT研究。
为了弄清1,1,1,3,3,3-六氟丙烷-2-醇(HFIP)的可能作用并提供溶液中的参考热化学数据,HFIP之间形成路易斯酸碱配合物(Lewis酸)和一系列8种不同的Lewis碱(3种亚砜,3种Nsp 2吡啶衍生物,1种芳族胺,1种环状脂族醚)通过等温滴定热(ITC)实验和通过分散(DFT- D)计算。测得的ITC缔合焓值(ΔH a)在-9.3和-14 kcal mol -1之间。与具有ΔH a的ITC数据相比,包括PCM隐式溶剂化模型在内的计算都对所有研究系统产生相似的放热关系。值介于-8.5至-12.7 kcal mol -1之间。一组额外的计算结合了反应物的氯苯的隐式和显式溶剂化,指出溶剂对HFIP-碱络合物的干扰相对较低:其主要作用是略微提高HFIP-刘易斯碱缔合的吉布斯能。据推测,本体HFIP与路易斯碱的相互作用因此不仅可以通过介质的动态微结构而且还可以通过影响路易斯碱处的键的极化而更明显地介入催化过程。










































 京公网安备 11010802027423号
京公网安备 11010802027423号