Microporous and Mesoporous Materials ( IF 4.8 ) Pub Date : 2020-07-03 , DOI: 10.1016/j.micromeso.2020.110409 Jianwen Liu , Wenzhi Luo , Hailin Cao , Luqian Weng , Gang Feng , Xian-Zhu Fu , Jing-Li Luo
|
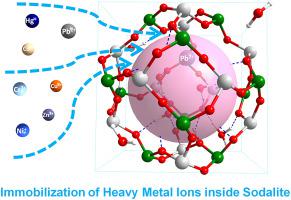
The geopolymer containing the special porous structure unit sod (t-toc) cage, which can be made from fly ash, is a possible economic solution to reduce toxicity of hazardous heavy metals by its excellent immobilization potential. Herein, the zeolite sodalite which is composed of sod (t-toc) cage was selected to model the geopolymer to uncover the immobilization mechanisms for the hazardous heavy metal ions Cr3+, Pb2+, Zn2+, Cu2+, Hg+, Cd2+ and Ni2+ in this work. Based on experimental crystalline structures, exchange energy was introduced for the first time to quantitatively evaluate the immobilization long-term stability theoretically. The immobilization of hazardous heavy metal ions were discovered to be determined by the solvation from the crystalline H2O and bonding from the sod (t-toc) cage. Based on the analysis of exchange energies, it was found that the immobilization for these heavy metals was correlated with the solvation radius: the larger the solvation radius was, the more favorable the immobilization was. Further detailed solvation effect analysis indicates that the immobilization of heavy metal ions is very sensitive to solvation effect based on the crystalline H2O molecules. Due to the synergistic effect between the solvation effect and the adsorption of the sod (t-toc) cage, the immobilization of Zn2+ is always favorable inside sodalite whereas Cr3+, Cd2+, Cu2+, Ni2+ and Pb2+ could be unfavorable in the case of insufficient solvation. This study provides a fast, convenient and objective way to evaluate the immobilization of heavy metals.
中文翻译:

从分子水平理解方钠石笼中有害重金属离子的固定机理:DFT研究
包含特殊的多孔结构单元草皮(t-toc)笼的地质聚合物,可以用粉煤灰制成,由于其极好的固定潜力,可以降低有害重金属的毒性,是一种可能的经济解决方案。在此,选择由草皮(t-toc)笼组成的沸石方钠石来模拟地聚合物,以揭示有害重金属离子Cr 3+,Pb 2 +,Zn 2 +,Cu 2 +,Hg的固定化机理。+,Cd 2+和Ni 2+在这项工作中。基于实验晶体结构,首次引入了交换能量,从理论上定量评估了固定化的长期稳定性。发现有害重金属离子的固定取决于结晶H 2 O的溶剂化和草皮(t-toc)笼的键合。基于交换能的分析,发现这些重金属的固定化与溶剂化半径相关:溶剂化半径越大,固定化越有利。进一步详细的溶剂化作用分析表明,重金属离子的固定化对基于结晶H 2的溶剂化作用非常敏感O分子。由于溶剂化作用与草皮(t-toc)笼的吸附之间具有协同作用,所以在方钠石内部固定Zn 2+总是有利的,而Cr 3+,Cd 2 +,Cu 2 +,Ni 2+和在溶剂化不足的情况下,Pb 2+可能是不利的。这项研究提供了一种快速,方便和客观的方法来评估重金属的固定化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号