Journal of Water Process Engineering ( IF 6.3 ) Pub Date : 2020-07-02 , DOI: 10.1016/j.jwpe.2020.101470 Huiru Hao , Qian Zhang , Yue Qiu , Li Meng , Xiaonan Wei , Wenjiao Sang , Jiawei Tao
|
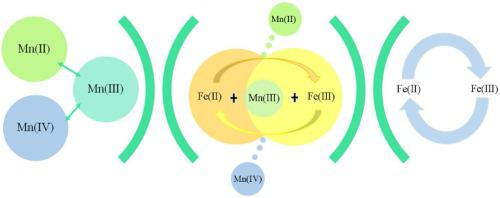
In this study, sludge-derived biochar (SDBC) loaded with metal oxides was successfully synthesized via a simple impregnation method, including monometallic-based SDBC (Fe, F-SDBC; Mn, M-SDBC) and bimetallic-based SDBC (Fe and Mn, FM-SDBC). Results demonstrated that FM-SDBC possessed higher activation capacity than F/M-SDBC. The degradation of Orange G (OG) under different pH were explored. Leaching of metal ions and cycling test showed that FM-SDBC possess extraordinary stability and reusability during repeated activation of persulfate (PS). It was evidenced by quenching studies that both SO4·- and ·OH were generated in FM-SDBC/PS. The XPS spectra of Fe 2p and Mn 2p before and after reactions indicated that the activation of PS on FM-SDBC was in company with the redox cycling of Fe and Mn species. Through the kinetics study, the contribution of Mn occupied the dominant position at the beginning, and Fe did in subsequent activation process in FM-SDBC/PS system. Synergistic mechanism between Fe and Mn in FM-SDBC could be attributed to (1) mutual electron transfer between Fe and Mn; (2) the formation of Fe-Mn bonds, which facilitated electron transfer between Fe and Mn to some extent. Based on the synergistic mechanism aforementioned, bimetallic-based biochar, especially FM-SDBC prepared in this study, had the benefits of high efficiency, stability, reusability and waste control by waste, which provided new insights into sulfate radical (SO4·-)-based advanced oxidation processes in practical application.
were generated in FM-SDBC/PS. The XPS spectra of Fe 2p and Mn 2p before and after reactions indicated that the activation of PS on FM-SDBC was in company with the redox cycling of Fe and Mn species. Through the kinetics study, the contribution of Mn occupied the dominant position at the beginning, and Fe did in subsequent activation process in FM-SDBC/PS system. Synergistic mechanism between Fe and Mn in FM-SDBC could be attributed to (1) mutual electron transfer between Fe and Mn; (2) the formation of Fe-Mn bonds, which facilitated electron transfer between Fe and Mn to some extent. Based on the synergistic mechanism aforementioned, bimetallic-based biochar, especially FM-SDBC prepared in this study, had the benefits of high efficiency, stability, reusability and waste control by waste, which provided new insights into sulfate radical (SO4·-)-based advanced oxidation processes in practical application.
中文翻译:

铁和锰氧化物改性生物炭活化过硫酸盐降解橙G的见解:铁和锰之间的协同作用
在这项研究中,通过简单的浸渍方法成功合成了负载有金属氧化物的污泥衍生生物炭(SDBC),包括单金属基SDBC(Fe,F-SDBC; Mn,M-SDBC)和双金属基SDBC(Fe和Mn,FM-SDBC)。结果表明,FM-SDBC具有比F / M-SDBC高的活化能力。探索了在不同pH条件下Orange G的降解。金属离子的浸出和循环测试表明,FM-SDBC在过硫酸盐(PS)的反复活化过程中具有非凡的稳定性和可重复使用性。淬火研究证明,SO 4 ·-和·OH 是在FM-SDBC / PS中生成的。反应前后Fe 2p和Mn 2p的XPS光谱表明,FM-SDBC上PS的活化与Fe和Mn物种的氧化还原循环有关。通过动力学研究,在FM-SDBC / PS系统中,Mn在开始时就占据了主导地位,Fe在随后的活化过程中占据了主导地位。FM-SDBC中Fe和Mn之间的协同作用机理可归因于(1)Fe和Mn之间的相互电子转移;(2)Fe-Mn键的形成,在一定程度上促进了Fe和Mn之间的电子转移。基于上述协同机制,本研究中制备的双金属基生物炭,尤其是FM-SDBC,具有高效,稳定,可重复使用和废物控制废物的优点,这为硫酸根自由基(SO4 ·-)为基础的先进氧化工艺在实际应用中。
是在FM-SDBC / PS中生成的。反应前后Fe 2p和Mn 2p的XPS光谱表明,FM-SDBC上PS的活化与Fe和Mn物种的氧化还原循环有关。通过动力学研究,在FM-SDBC / PS系统中,Mn在开始时就占据了主导地位,Fe在随后的活化过程中占据了主导地位。FM-SDBC中Fe和Mn之间的协同作用机理可归因于(1)Fe和Mn之间的相互电子转移;(2)Fe-Mn键的形成,在一定程度上促进了Fe和Mn之间的电子转移。基于上述协同机制,本研究中制备的双金属基生物炭,尤其是FM-SDBC,具有高效,稳定,可重复使用和废物控制废物的优点,这为硫酸根自由基(SO4 ·-)为基础的先进氧化工艺在实际应用中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号