Journal of Pharmaceutical Analysis ( IF 6.1 ) Pub Date : 2020-07-03 , DOI: 10.1016/j.jpha.2020.06.007 Minshan Shou , Haixiao Qiu
|
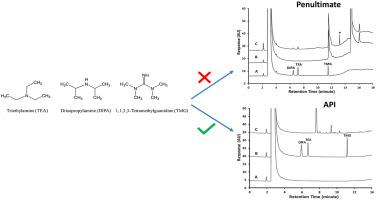
A rapid GC-FID method was developed to simultaneously determine residual levels of triethylamine (TEA), 1,1,3,3-tetramethylguanidine (TMG), and diisopropylamine (DIPA) in the synthetic route of an active pharmaceutical ingredient (API). Due to the severe absorption of amines on GC stationary phases, GC columns with various stationary phases were evaluated for optimal peak shape and reproducibility. The final conditions used the Agilent CP-Volamine column to resolve the three amines in 12 min. Various inlet liners were also screened to further improve the sensitivity of the analysis. The Restek Siltek® liner was selected to achieve the desired detectability for the method. The quantitation limits were 4, 3, and 4 μg/mL for TEA, DIPA, and TMG in the presence of API, respectively. All three amines showed good linearity (r > 0.999) and recoveries (> 90%) over the concentration range of 3 to 16 μg/mL. The testing of residual amines was initially performed at the penultimate stage of the synthesis. However, this work demonstrates that TMG can act as a proton sponge to react with salicylic acid, the counter ion of the penultimate, to form a volatile component that elutes at a different retention time. Consequently, in the final method, these three amines were monitored in the final API to circumvent the matrix interference. Key parameters of the method were qualified per method validation requirements in ICH guidelines. The method was successfully applied for batch testing during development and implemented as an in-process control procedure at manufacturing sites.
中文翻译:

快速GC-FID方法的开发可同时测定活性药物成分中的三乙胺,二异丙胺和1,1,3,3-四甲基胍残基
开发了一种快速GC-FID方法,以同时测定活性药物成分(API)合成途径中的三乙胺(TEA),1,1,3,3-四甲基胍(TMG)和二异丙胺(DIPA)的残留量。由于胺在GC固定相上的吸收严重,因此对具有各种固定相的GC色谱柱进行了最佳峰形和重现性的评估。最终条件使用Agilent CP-Volamine色谱柱在12分钟内分离出三种胺。还对各种进样口衬管进行了筛选,以进一步提高分析的灵敏度。选择RestekSiltek®衬管以实现该方法所需的可检测性。在API存在下,TEA,DIPA和TMG的定量限分别为4、3和4μg/ mL。所有三种胺均显示出良好的线性(r > 0.999)并在3至16μg/ mL的浓度范围内回收(> 90%)。残留胺的测试最初是在合成的倒数第二阶段进行的。但是,这项工作表明TMG可以充当质子海绵,与倒数第二个水杨酸的水杨酸反应,形成挥发性组分,并在不同的保留时间洗脱。因此,在最终方法中,在最终API中对这三种胺进行了监测,以规避基质干扰。该方法的关键参数均按照ICH指南中的方法验证要求进行了限定。该方法已成功应用于开发过程中的批处理测试,并已在制造现场作为过程中的控制程序实施。











































 京公网安备 11010802027423号
京公网安备 11010802027423号