Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2020-07-03 , DOI: 10.1016/j.jinorgbio.2020.111174 Surbhi Jain 1 , Kishalay Bhar 1 , Shreetama Bandyopadhayaya 2 , Vikas K Singh 3 , Chandi C Mandal 2 , Suman Tapryal 3 , Anuj K Sharma 1
|
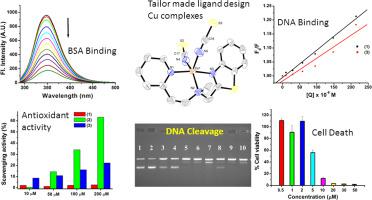
Research on development of novel metal based anti-cancer agents continues with its popularity among bioinorganic community. Benzothiazole, an important heterocyclic pharmacophore, was chosen as a valuable and useful scaffold for the synthesis of novel copper(II) complexes. Three new copper(II) complexes obtained from the synthesis of newly synthesized benzothiazole based N-(benzo[d]thiazol-2-ylmethyl)-N-methyl-2-(pyridin-2-yl)ethan-1-amine (btzpy) ligand with CuCl2 [Cu(btzpy)Cl2] (1), Cu(NCS)2 [Cu(btzpy)(NCS)2] (2), and Cu(NO3)2 [Cu(btzpy)(NO3)(H2O)]NO3 (3) were isolated and characterized by physical and spectroscopic measurements, including single-crystal X-ray structures. The interaction of complexes 1 and 3 with calf thymus (CT)-DNA was investigated using ethidium bromide fluorescence quenching assay and weak intercalation with KSV values of 9.8 × 102 M−1 and 8.2 × 102 M−1, respectively was observed. All three complexes have shown DNA cleavage of supercoiled plasmid DNA forming single nicked and double nicked forms in the presence of external reducing agents like 3-mercaptopropionic acid (3-MPA) and ascorbic acid. The water-soluble complexes 1 and 3 also show prominent hydrolytic DNA cleavage. From the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay, it was observed that complex 2 also exhibits good antioxidant properties. The cytotoxicity of complexes 1–3 was tested against the lung cancer cell line (A549) and complex 2 with -NCS moiety shows maximum activity in the micromolar range. A rationale for the observed activity is proposed in light of the other properties of these molecules.
中文翻译:

阴离子共配体的开发,评价和对苯并噻唑衍生的铜(II)配合物的生物活性的影响。
随着新型金属基抗癌药在生物无机界的普及,其研究仍在继续。苯并噻唑是一种重要的杂环药效团,被选为合成新型铜(II)配合物的有价值和有用的支架。从新合成的基于苯并噻唑的N-(苯并[ d ]噻唑-2-基甲基)-N-甲基-2-(吡啶-2-基)乙-1-胺(btzpy)的合成中获得的三种新的铜(II)配合物)与CuCl 2 [Cu(btzpy)Cl 2 ](1),Cu(NCS)2 [Cu(btzpy)(NCS)2 ](2)和Cu(NO3)2 [Cu(btzpy)(NO 3)(H 2 O)] NO 3(3)的分离和特征在于物理和光谱测量,包括单晶X射线结构。使用溴化乙锭荧光猝灭法和弱插层法研究了配合物1和3与小牛胸腺(CT)-DNA的相互作用,K SV值为9.8×10 2 M -1和8.2×10 2 M -1,分别被观察到。在存在外部还原剂(例如3-巯基丙酸(3-MPA)和抗坏血酸)的情况下,所有三种复合物均显示超螺旋质粒DNA的DNA切割形成单切口和双切口形式。水溶性复合物1和3也显示出显着的水解DNA裂解。从DPPH(2,2-二苯基-1-吡啶并肼基)自由基清除测定中,观察到配合物2也表现出良好的抗氧化性能。复合物的细胞毒性1 - 3中针对肺癌细胞系(A549)和复杂的测试2具有-NCS部分的R 2在微摩尔范围内显示最大活性。根据这些分子的其他性质,提出了观察到的活性的基本原理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号