当前位置:
X-MOL 学术
›
Colloids Surf. A Physicochem. Eng. Aspects
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Two Co(Ⅱ)-Based Metal Organic Frameworks for Highly Efficient Removal of Azo Dyes from Aqueous Environment: Synthesis, Selective Adsorption and Adsorption Mechanism
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.colsurfa.2020.125236 Wenbo Liu , Chuanbin Fan , Ziao Zong , Nana Li , Kaixuan Ma , Bin Zhu , Xia Zhang , Yuhua Fan
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.colsurfa.2020.125236 Wenbo Liu , Chuanbin Fan , Ziao Zong , Nana Li , Kaixuan Ma , Bin Zhu , Xia Zhang , Yuhua Fan
|
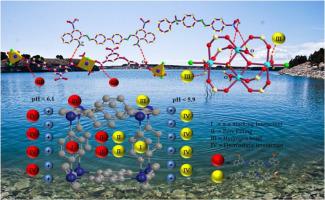
Abstract Two novel Co(Ⅱ)-MOFs synthesized under solvothermal conditions, namely [Co3(L)2(bimb)2(H2O)2]n (OUC-1) and [Co2(L)(OH)(H2O)2]n (OUC-2) (bimb = 1,4-bis(lmidazol)butane, H3L = 3-(3,5-dicarboxylphenoxy)-5-carboxylpyridine), were used to remove Congo Red and Orange Ⅳ from aqueous environment, respectively. The influential parameters of the temperature and pH of solution, initial dye concentration, the structure and charge of dye on the adsorption performance were investigated. It is found that the dye with proper structure (i.e. organic groups favorable for adsorption, such as -NH2, -SO3H, etc, proper distance between the groups, linear structure and more benzene rings), richer negative charges, smaller molecular weight facilitates faster adsorbed. The intermittent operation experiments were adopted to make the adsorption isotherm and kinetics data of OUC-1 and OUC-2 accurately described by Langmuir isotherm and pseudo second-order kinetics. The intra-particle diffusion model indicates the adsorption process is controlled by the intra-particle diffusion and other adsorption stages. Two adsorbents release azo dyes easily through simple organic solutions and maintain high adsorption capacity after 8 cycles. Moreover, OUC-1 and OUC-2 also exhibit high adsorption performance in artificial seawater. The mechanism of selective adsorption involves pore filling, π-π stacking interaction, hydrogen bond and electrostatic interaction.
中文翻译:

用于高效去除水性环境中偶氮染料的两种 Co(II) 基金属有机骨架:合成、选择性吸附和吸附机制
摘要 在溶剂热条件下合成了两种新型 Co(Ⅱ)-MOFs,即 [Co3(L)2(bimb)2(H2O)2]n (OUC-1) 和 [Co2(L)(OH)(H2O)2] n (OUC-2)(bimb = 1,4-双(咪唑)丁烷,H3L = 3-(3,5-二羧基苯氧基)-5-羧基吡啶),分别用于从水环境中去除刚果红和橙Ⅳ . 考察了溶液的温度和pH值、初始染料浓度、染料的结构和电荷对吸附性能的影响参数。发现具有适当结构的染料(即有利于吸附的有机基团,如-NH2、-SO3H等,基团间距适当,呈线性结构,苯环较多),负电荷较多,分子量较小,吸附速度较快吸附。采用间歇操作实验,使OUC-1和OUC-2的吸附等温线和动力学数据用Langmuir等温线和准二级动力学准确描述。颗粒内扩散模型表明吸附过程受颗粒内扩散和其他吸附阶段的控制。两种吸附剂通过简单的有机溶液轻松释放偶氮染料,并在 8 个循环后保持高吸附容量。此外,OUC-1和OUC-2在人工海水中也表现出高吸附性能。选择性吸附的机理涉及孔隙填充、π-π堆积相互作用、氢键和静电相互作用。颗粒内扩散模型表明吸附过程受颗粒内扩散和其他吸附阶段的控制。两种吸附剂通过简单的有机溶液轻松释放偶氮染料,并在 8 个循环后保持高吸附容量。此外,OUC-1和OUC-2在人工海水中也表现出高吸附性能。选择性吸附的机理涉及孔隙填充、π-π堆积相互作用、氢键和静电相互作用。颗粒内扩散模型表明吸附过程受颗粒内扩散和其他吸附阶段的控制。两种吸附剂通过简单的有机溶液轻松释放偶氮染料,并在 8 个循环后保持高吸附容量。此外,OUC-1和OUC-2在人工海水中也表现出高吸附性能。选择性吸附的机理涉及孔隙填充、π-π堆积相互作用、氢键和静电相互作用。
更新日期:2020-10-01
中文翻译:

用于高效去除水性环境中偶氮染料的两种 Co(II) 基金属有机骨架:合成、选择性吸附和吸附机制
摘要 在溶剂热条件下合成了两种新型 Co(Ⅱ)-MOFs,即 [Co3(L)2(bimb)2(H2O)2]n (OUC-1) 和 [Co2(L)(OH)(H2O)2] n (OUC-2)(bimb = 1,4-双(咪唑)丁烷,H3L = 3-(3,5-二羧基苯氧基)-5-羧基吡啶),分别用于从水环境中去除刚果红和橙Ⅳ . 考察了溶液的温度和pH值、初始染料浓度、染料的结构和电荷对吸附性能的影响参数。发现具有适当结构的染料(即有利于吸附的有机基团,如-NH2、-SO3H等,基团间距适当,呈线性结构,苯环较多),负电荷较多,分子量较小,吸附速度较快吸附。采用间歇操作实验,使OUC-1和OUC-2的吸附等温线和动力学数据用Langmuir等温线和准二级动力学准确描述。颗粒内扩散模型表明吸附过程受颗粒内扩散和其他吸附阶段的控制。两种吸附剂通过简单的有机溶液轻松释放偶氮染料,并在 8 个循环后保持高吸附容量。此外,OUC-1和OUC-2在人工海水中也表现出高吸附性能。选择性吸附的机理涉及孔隙填充、π-π堆积相互作用、氢键和静电相互作用。颗粒内扩散模型表明吸附过程受颗粒内扩散和其他吸附阶段的控制。两种吸附剂通过简单的有机溶液轻松释放偶氮染料,并在 8 个循环后保持高吸附容量。此外,OUC-1和OUC-2在人工海水中也表现出高吸附性能。选择性吸附的机理涉及孔隙填充、π-π堆积相互作用、氢键和静电相互作用。颗粒内扩散模型表明吸附过程受颗粒内扩散和其他吸附阶段的控制。两种吸附剂通过简单的有机溶液轻松释放偶氮染料,并在 8 个循环后保持高吸附容量。此外,OUC-1和OUC-2在人工海水中也表现出高吸附性能。选择性吸附的机理涉及孔隙填充、π-π堆积相互作用、氢键和静电相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号