Clinical Gastroenterology and Hepatology ( IF 11.6 ) Pub Date : 2020-07-03 , DOI: 10.1016/j.cgh.2020.06.050 Parakkal Deepak 1 , Quazim A Alayo 2 , Aava Khatiwada 1 , Bixuan Lin 3 , Marc Fenster 4 , Christina Dimopoulos 5 , Geoffrey Bader 6 , Roni Weisshof 7 , Michael Jacobs 7 , Alexandra Gutierrez 1 , Matthew A Ciorba 1 , George P Christophi 8 , Anish Patel 6 , Robert P Hirten 5 , Jean-Frederic Colombel 5 , David T Rubin 7 , Christina Ha 9 , Poonam Beniwal-Patel 3 , Ryan C Ungaro 5 , Gaurav Syal 9 , Joel Pekow 7 , Benjamin L Cohen 10 , Andres Yarur 3
|
Background & Aims
Adverse events (AEs) including reactivation of herpes zoster (HZ) and venous thromboembolism (VTE) have been reported from clinical trials of tofacitinib in ulcerative colitis (UC). We investigated the incidence rates of AEs in a real-world study of UC patients given tofacitinib.
Methods
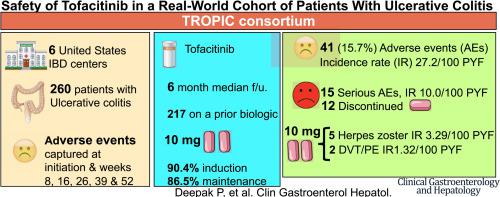
We collected data from 260 patients with UC in the Tofacitinib Real-world Outcomes in Patients with ulceratIve colitis and Crohn’s disease consortium study, performed at 6 medical centers in the United States. Patients were followed up for a median of 6 months (interquartile range, 2.7–11.5 mo). AEs were captured using a standardized data collection instrument before study initiation and at weeks 8, 16, 26, 39, and 52. Serious AEs were defined as life-threatening or resulting in a hospitalization, disability, or discontinuation of therapy. Logistic regression was performed to examine risk factors for AEs.
Results
AEs occurred in 41 patients (15.7%); most were infections (N = 13; 5.0%). The incidence rate of any AE was 27.2 (95% CI, 24.4–30.7 per 100 patient-years of follow-up evaluation). Fifteen were serious AEs (36.6% of AEs), and tofacitinib was discontinued for 12 patients (4.6% of cohort). The incidence rates of serious AEs was 10.0 (95% CI, 8.9–11.2 per 100 patient-years of follow-up evaluation). Five patients developed HZ infection and 2 developed VTE (all receiving 10 mg tofacitinib, twice per day).
Conclusions
Real-world safety signals for tofacitinib are similar to those for clinical trials, with AEs reported from almost 16% of patients. HZ infection and VTE occurred in patients receiving 10 mg tofacitinib twice per day. These results support dose de-escalation after induction therapy, to reduce the risk of AEs.
中文翻译:

托法替尼在溃疡性结肠炎患者真实世界队列中的安全性
背景与目标
托法替尼治疗溃疡性结肠炎 (UC) 的临床试验报告了包括带状疱疹 (HZ) 再激活和静脉血栓栓塞 (VTE) 在内的不良事件 (AE)。我们调查了给予托法替尼的 UC 患者的真实世界研究中 AE 的发生率。
方法
我们在美国 6 个医疗中心进行的 Tofacitinib 溃疡性结肠炎患者和克罗恩病联合研究的真实世界结果中收集了 260 名 UC 患者的数据。患者的中位随访时间为 6 个月(四分位距,2.7-11.5 个月)。在研究开始前和第 8、16、26、39 和 52 周时使用标准化数据收集工具捕获 AE。严重 AE 被定义为危及生命或导致住院、残疾或停止治疗。进行逻辑回归以检查 AE 的风险因素。
结果
41 名患者 (15.7%) 发生 AE;大多数是感染(N = 13;5.0%)。任何 AE 的发生率为 27.2(95% CI,24.4-30.7/100 患者年的随访评估)。15 例为严重 AE(占 AE 的 36.6%),12 例患者(占队列的 4.6%)停用托法替尼。严重 AE 的发生率为 10.0(95% CI,8.9-11.2/100 患者年的随访评估)。5 名患者出现 HZ 感染,2 名出现 VTE(均接受 10 mg 托法替尼,每天两次)。
结论
托法替尼的真实世界安全信号与临床试验相似,几乎 16% 的患者报告了 AE。每天两次接受 10 mg 托法替尼的患者发生 HZ 感染和 VTE。这些结果支持诱导治疗后降低剂量,以降低 AE 的风险。











































 京公网安备 11010802027423号
京公网安备 11010802027423号