Cell ( IF 45.5 ) Pub Date : 2020-07-02 , DOI: 10.1016/j.cell.2020.05.049 Leandro Venturutti 1 , Matt Teater 1 , Andrew Zhai 2 , Amy Chadburn 3 , Leena Babiker 1 , Daleum Kim 1 , Wendy Béguelin 1 , Tak C Lee 1 , Youngjun Kim 4 , Christopher R Chin 5 , William T Yewdell 6 , Brian Raught 7 , Jude M Phillip 1 , Yanwen Jiang 1 , Louis M Staudt 8 , Michael R Green 9 , Jayanta Chaudhuri 10 , Olivier Elemento 11 , Pedro Farinha 12 , Andrew P Weng 13 , Michael D Nissen 14 , Christian Steidl 12 , Ryan D Morin 12 , David W Scott 12 , Gilbert G Privé 15 , Ari M Melnick 1
|
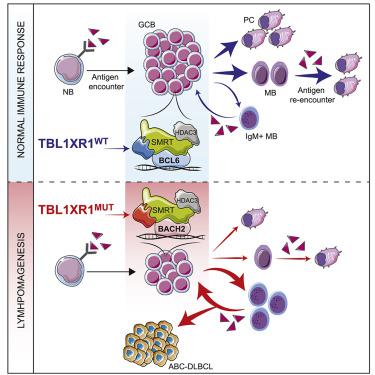
The most aggressive B cell lymphomas frequently manifest extranodal distribution and carry somatic mutations in the poorly characterized gene TBL1XR1. Here, we show that TBL1XR1 mutations skew the humoral immune response toward generating abnormal immature memory B cells (MB), while impairing plasma cell differentiation. At the molecular level, TBL1XR1 mutants co-opt SMRT/HDAC3 repressor complexes toward binding the MB cell transcription factor (TF) BACH2 at the expense of the germinal center (GC) TF BCL6, leading to pre-memory transcriptional reprogramming and cell-fate bias. Upon antigen recall, TBL1XR1 mutant MB cells fail to differentiate into plasma cells and instead preferentially reenter new GC reactions, providing evidence for a cyclic reentry lymphomagenesis mechanism. Ultimately, TBL1XR1 alterations lead to a striking extranodal immunoblastic lymphoma phenotype that mimics the human disease. Both human and murine lymphomas feature expanded MB-like cell populations, consistent with a MB-cell origin and delineating an unforeseen pathway for malignant transformation of the immune system.
中文翻译:

TBL1XR1突变通过诱导肿瘤发生前记忆命运驱动结外淋巴瘤。
最具攻击性的B细胞淋巴瘤经常表现出结外分布,并在特征不明确的基因TBL1XR1中携带体细胞突变。在这里,我们显示TBL1XR1突变会使体液免疫反应偏向于产生异常的未成熟记忆B细胞(MB),同时损害浆细胞分化。在分子水平上,TBL1XR1突变体共同选择SMRT / HDAC3阻遏物复合物以结合MB细胞转录因子(TF)BACH2,而以生发中心(GC)TF BCL6为代价,从而导致内存前转录重编程和细胞命运偏压。抗原召回后,TBL1XR1突变型MB细胞无法分化为浆细胞,而是优先重新进入新的GC反应,从而为循环再进入淋巴瘤发生机制提供了证据。最终,TBL1XR1这种改变会导致惊人的结外免疫母细胞淋巴瘤表型,模仿人类疾病。人淋巴瘤和鼠淋巴瘤均具有扩大的MB样细胞群,与MB细胞起源一致,并描绘了免疫系统恶性转化的不可预见的途径。









































 京公网安备 11010802027423号
京公网安备 11010802027423号