Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-07-03 , DOI: 10.1016/j.bmc.2020.115623 Mei Zhu 1 , Ling Ma 1 , Biao Dong 1 , Guoning Zhang 1 , Juxian Wang 1 , Jinming Zhou 2 , Shan Cen 1 , Yucheng Wang 1
|
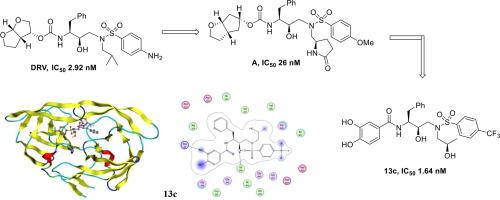
Newly designed HIV-1 protease inhibitors that maximize interactions with the protein backbone, especially in the form of hydrogen bonds, may enhance the antiviral potency of these compounds and minimize acquisition of drug-resistant mutations. Herein, we described a series of new HIV-1 PIs containing phenols as the P2 ligands and chiral isopropanol as the P1′ ligands, in combination with 4-trifluoromethylphenylsulfonamide or 4-nitrophenylsulfonamide as the P2′ ligands. And most of these compounds exhibited nanomolar inhibitory potency. In particular, inhibitors 13c and 13e with 4-trifluoromethylphenylsulfonamide as the P2′ ligand and (R) – isopropanol as the P1′ ligand, exhibited antiviral IC50 values of 1.64 nM and 2.33 nM, respectively. Furthermore, they also showed remarkable activity against wild-type and DRV-resistant HIV-1 variants that raised the prospect of designing more effective PIs further.
中文翻译:

以异丙醇为P1'配体以增强与S1'子位点的结合的新型HIV-1蛋白酶抑制剂的设计和生物学评估。
新设计的HIV-1蛋白酶抑制剂可以最大程度地与蛋白质骨架相互作用,尤其是以氢键形式,可以增强这些化合物的抗病毒效力,并最小化耐药突变的产生。在本文中,我们描述了一系列新的HIV-1 PI,它们含有酚作为P 2配体和手性异丙醇作为P 1'配体,并与4-三氟甲基苯基磺酰胺或4-硝基苯基磺酰胺作为P 2'配体结合。而且大多数这些化合物表现出纳摩尔抑制能力。尤其是抑制剂13c和13e,其中4-三氟甲基苯基磺酰胺为P 2'配体,而(R)–异丙醇作为P 1'配体,其抗病毒IC 50值分别为1.64 nM和2.33 nM。此外,他们还表现出了针对野生型和抗DRV的HIV-1变体的显着活性,这为进一步设计更有效的PI带来了希望。











































 京公网安备 11010802027423号
京公网安备 11010802027423号