当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular crowding effects on the biochemical properties of amyloid β–heme, Aβ–Cu and Aβ–heme–Cu complexes
Chemical Science ( IF 7.6 ) Pub Date : 2020-07-02 , DOI: 10.1039/d0sc01020k Meng Li 1, 2, 3, 4, 5 , Zhenqi Liu 1, 2, 3, 4, 5 , Jinsong Ren 1, 2, 3, 4, 5 , Xiaogang Qu 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2020-07-02 , DOI: 10.1039/d0sc01020k Meng Li 1, 2, 3, 4, 5 , Zhenqi Liu 1, 2, 3, 4, 5 , Jinsong Ren 1, 2, 3, 4, 5 , Xiaogang Qu 1, 2, 3, 4, 5
Affiliation
|
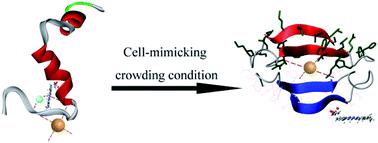
Heme as a cofactor has been proposed to bind with β-amyloid peptide (Aβ) and the formed Aβ–heme complex exhibits enhanced peroxidase-like activity. So far, in vitro studies on the interactions between heme, Cu and Aβ have been exclusively performed in dilute solution. However, the intracellular environment is highly crowded with biomolecules. Therefore, exploring how Aβ–heme–Cu complexes behave under molecular crowding conditions is critical for understanding the mechanism of Aβ neurotoxicity in vivo. Herein, we selected PEG-200 as a crowding agent to mimic the crowded cytoplasmic environment for addressing the contributions of crowded physiological environments to the biochemical properties of Aβ–heme, Aβ–Cu and Aβ–heme–Cu complexes. Surprisingly, experimental studies and theoretical calculations revealed that molecular crowding weakened the stabilization of the Aβ–heme complex and decreased its peroxidase activity. Our data attributed this consequence to the decreased binding affinity of heme to Aβ as a result of the alterations in water activity and Aβ conformation. Our findings highlight the significance of hydration effects on the interaction of Aβ–heme and Aβ–Cu and their peroxidase activities. Molecular crowding inside cells may potentially impose a positive effect on Aβ–Cu but a negative effect on the interaction of Aβ with heme. This indicates that Aβ40–Cu but not Aβ40–heme may play more important roles in the oxidative damage in the etiology of AD. Therefore, this work provides a new clue for understanding the oxidative damage occurring in AD.
中文翻译:

分子拥挤对淀粉样β-血红素,Aβ-Cu和Aβ-血红素-Cu复合物生化特性的影响
已提出血红素作为辅助因子可与β-淀粉样肽(Aβ)结合,并且形成的Aβ-血红素复合物表现出增强的过氧化物酶样活性。迄今为止,仅在稀溶液中进行了血红素,铜和Aβ之间相互作用的体外研究。然而,细胞内环境高度拥挤有生物分子。因此,探索Aβ-血红素-Cu配合物在分子拥挤条件下的行为对于了解体内Aβ神经毒性的机制至关重要。。在这里,我们选择PEG-200作为一种拥挤剂来模拟拥挤的细胞质环境,以解决拥挤的生理环境对Aβ-血红素,Aβ-Cu和Aβ-血红素-Cu复合物的生化特性的影响。令人惊讶的是,实验研究和理论计算表明,分子拥挤削弱了Aβ-血红素复合物的稳定性,并降低了其过氧化物酶活性。我们的数据将此结果归因于水活度和Aβ构象的改变导致血红素与Aβ的结合亲和力降低。我们的发现突出了水合作用对Aβ-血红素和Aβ-Cu相互作用及其过氧化物酶活性的重要性。细胞内的分子拥挤可能会对Aβ-Cu产生正面影响,但对Aβ与血红素的相互作用产生负面影响。这表明在AD的病因中,Aβ40-Cu而不是Aβ40-血红素可能在氧化损伤中起更重要的作用。因此,这项工作为理解AD中发生的氧化损伤提供了新的线索。
更新日期:2020-07-22
中文翻译:

分子拥挤对淀粉样β-血红素,Aβ-Cu和Aβ-血红素-Cu复合物生化特性的影响
已提出血红素作为辅助因子可与β-淀粉样肽(Aβ)结合,并且形成的Aβ-血红素复合物表现出增强的过氧化物酶样活性。迄今为止,仅在稀溶液中进行了血红素,铜和Aβ之间相互作用的体外研究。然而,细胞内环境高度拥挤有生物分子。因此,探索Aβ-血红素-Cu配合物在分子拥挤条件下的行为对于了解体内Aβ神经毒性的机制至关重要。。在这里,我们选择PEG-200作为一种拥挤剂来模拟拥挤的细胞质环境,以解决拥挤的生理环境对Aβ-血红素,Aβ-Cu和Aβ-血红素-Cu复合物的生化特性的影响。令人惊讶的是,实验研究和理论计算表明,分子拥挤削弱了Aβ-血红素复合物的稳定性,并降低了其过氧化物酶活性。我们的数据将此结果归因于水活度和Aβ构象的改变导致血红素与Aβ的结合亲和力降低。我们的发现突出了水合作用对Aβ-血红素和Aβ-Cu相互作用及其过氧化物酶活性的重要性。细胞内的分子拥挤可能会对Aβ-Cu产生正面影响,但对Aβ与血红素的相互作用产生负面影响。这表明在AD的病因中,Aβ40-Cu而不是Aβ40-血红素可能在氧化损伤中起更重要的作用。因此,这项工作为理解AD中发生的氧化损伤提供了新的线索。











































 京公网安备 11010802027423号
京公网安备 11010802027423号