当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ordered Solvents and Ionic Liquids Can be Harnessed for Electrostatic Catalysis
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-07-01 , DOI: 10.1021/jacs.0c05643 Longkun Xu 1 , Ekaterina I Izgorodina 2 , Michelle L Coote 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-07-01 , DOI: 10.1021/jacs.0c05643 Longkun Xu 1 , Ekaterina I Izgorodina 2 , Michelle L Coote 1
Affiliation
|
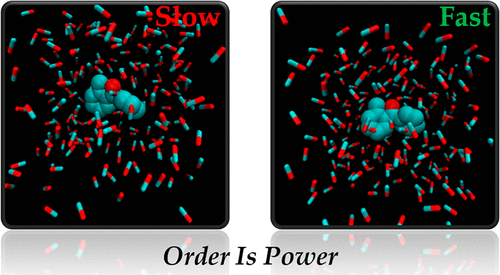
Herein we employ classical molecular dynamics simulations using the Drude oscillator based polarizable force field, quantum chemical calculations, and ONIOM multiscale calculations to study a) how an external field orders the solvent environment in a chemical reaction and then b) whether, in the absence of this same applied field, the ordered solvent environment alone can electrostatically catalyse a chemical reaction when compared with the corresponding disordered solvent. Our results show that a 0.2 V/Å external electric field, which is below the threshold for bond breaking of solvent molecules, leads to significant ordering of bulk methanol solvent and the ionic liquid [EMIM][BF4]. Importantly, in the absence of this same field, the ordered solvent lowers the activation energy of the hydrogen-transfer reaction of o-alkylphenyl ketones in excess of 20 kcal/mol when the solvent is methanol, and by over 30 kcal/mol for [EMIM][BF4]. Even a 0.1 V/Å external field has effects of ca. 10 and 20 kcal/mol respectively. This work suggests a possible strategy for scaling electrostatic catalysis by applying a pulsed external field to the reaction medium to maintain solvent ordering, while allowing the reaction to proceed largely in the absence of an external field.
中文翻译:

有序溶剂和离子液体可用于静电催化
在此,我们使用基于 Drude 振荡器的极化力场、量子化学计算和 ONIOM 多尺度计算采用经典分子动力学模拟来研究 a) 外部场如何在化学反应中对溶剂环境进行排序,然后 b) 在没有在相同的应用场中,与相应的无序溶剂相比,仅有序溶剂环境就可以静电催化化学反应。我们的结果表明,低于溶剂分子键断裂阈值的 0.2 V/Å 外部电场导致大量甲醇溶剂和离子液体 [EMIM][BF4] 的显着排序。重要的是,在没有相同领域的情况下,当溶剂为甲醇时,有序溶剂将邻烷基苯基酮的氢转移反应的活化能降低超过 20 kcal/mol,对于 [EMIM][BF4],降低超过 30 kcal/mol。即使是 0.1 V/Å 的外场也有大约 分别为 10 和 20 kcal/mol。这项工作提出了一种可能的策略,通过向反应介质施加脉冲外场以保持溶剂有序,同时允许反应在没有外场的情况下大量进行,从而扩大静电催化的规模。
更新日期:2020-07-01
中文翻译:

有序溶剂和离子液体可用于静电催化
在此,我们使用基于 Drude 振荡器的极化力场、量子化学计算和 ONIOM 多尺度计算采用经典分子动力学模拟来研究 a) 外部场如何在化学反应中对溶剂环境进行排序,然后 b) 在没有在相同的应用场中,与相应的无序溶剂相比,仅有序溶剂环境就可以静电催化化学反应。我们的结果表明,低于溶剂分子键断裂阈值的 0.2 V/Å 外部电场导致大量甲醇溶剂和离子液体 [EMIM][BF4] 的显着排序。重要的是,在没有相同领域的情况下,当溶剂为甲醇时,有序溶剂将邻烷基苯基酮的氢转移反应的活化能降低超过 20 kcal/mol,对于 [EMIM][BF4],降低超过 30 kcal/mol。即使是 0.1 V/Å 的外场也有大约 分别为 10 和 20 kcal/mol。这项工作提出了一种可能的策略,通过向反应介质施加脉冲外场以保持溶剂有序,同时允许反应在没有外场的情况下大量进行,从而扩大静电催化的规模。



























 京公网安备 11010802027423号
京公网安备 11010802027423号