当前位置:
X-MOL 学术
›
Aging Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The inherited methylome landscape is directly altered with paternal aging and associated with offspring neurodevelopmental disorders.
Aging Cell ( IF 8.0 ) Pub Date : 2020-07-01 , DOI: 10.1111/acel.13178 Michelle M Denomme 1 , Mary E Haywood 2 , Jason C Parks 1 , William B Schoolcraft 3 , Mandy G Katz-Jaffe 1, 2, 3
Aging Cell ( IF 8.0 ) Pub Date : 2020-07-01 , DOI: 10.1111/acel.13178 Michelle M Denomme 1 , Mary E Haywood 2 , Jason C Parks 1 , William B Schoolcraft 3 , Mandy G Katz-Jaffe 1, 2, 3
Affiliation
|
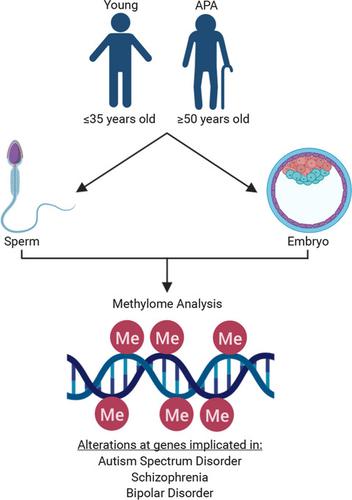
Paternal aging and the prevalence of neurodevelopmental disorders in offspring are well documented. Yet, the underlying mechanism and the mode of inheritance have not been conclusively established. Advancing paternal age is a subtle and varying phenotype. As such, it is likely that a threshold for cumulative risk may exist that, if surpassed, culminates in a predisposition to disease and ultimately an observed phenotype in offspring. Epigenetic regulation provides a plausible explanation for the nongenetic paternal transmission of disease susceptibility. With the use of whole‐genome methylation sequencing, the data described herein substantiate an increasingly compromised DNA methylation profile as sperm ages and, for the first time, also demonstrate a generational correlation in sperm and blastocyst of an altered methylome associated with advanced paternal age. Methylation alterations are not randomly distributed across the genome, but appear clustered at certain chromosomal locations, and significantly colocalize with regions of nucleosome retention. Genes associated with autism spectrum disorder, schizophrenia, and bipolar disorder are significantly enriched with causative methylation aberrations in both sperm and embryos from aged fathers. The long‐term health burden and societal economic impact of these conditions are substantial and will continue with increasingly prevalent diagnosis. This work provides a mechanistic link between the paternal age effect and offspring neurodevelopmental disorders leading to a better understanding of causation and investigation into potential future therapy.
中文翻译:

遗传的甲基化组景观会随着父亲的衰老而直接改变,并与后代的神经发育障碍有关。
父亲的衰老和后代神经发育障碍的患病率有据可查。然而,潜在的机制和继承方式尚未最终确定。父亲年龄的提前是一种微妙而多变的表型。因此,可能存在累积风险的阈值,如果超过该阈值,最终导致易患疾病并最终在后代中观察到表型。表观遗传调控为疾病易感性的非遗传父系传播提供了合理的解释。随着全基因组甲基化测序的使用,本文描述的数据证实了随着精子年龄的增长,DNA 甲基化谱越来越受到损害,并且第一次,还证明了与高龄父亲相关的甲基化组改变的精子和胚泡的代际相关性。甲基化改变不是随机分布在整个基因组中,而是聚集在某些染色体位置,并与核小体保留区域显着共存。与自闭症谱系障碍、精神分裂症和双相情感障碍相关的基因在年老父亲的精子和胚胎中显着富集了致病性甲基化畸变。这些疾病的长期健康负担和社会经济影响是巨大的,并且会随着诊断的日益普遍而继续。
更新日期:2020-07-01
中文翻译:

遗传的甲基化组景观会随着父亲的衰老而直接改变,并与后代的神经发育障碍有关。
父亲的衰老和后代神经发育障碍的患病率有据可查。然而,潜在的机制和继承方式尚未最终确定。父亲年龄的提前是一种微妙而多变的表型。因此,可能存在累积风险的阈值,如果超过该阈值,最终导致易患疾病并最终在后代中观察到表型。表观遗传调控为疾病易感性的非遗传父系传播提供了合理的解释。随着全基因组甲基化测序的使用,本文描述的数据证实了随着精子年龄的增长,DNA 甲基化谱越来越受到损害,并且第一次,还证明了与高龄父亲相关的甲基化组改变的精子和胚泡的代际相关性。甲基化改变不是随机分布在整个基因组中,而是聚集在某些染色体位置,并与核小体保留区域显着共存。与自闭症谱系障碍、精神分裂症和双相情感障碍相关的基因在年老父亲的精子和胚胎中显着富集了致病性甲基化畸变。这些疾病的长期健康负担和社会经济影响是巨大的,并且会随着诊断的日益普遍而继续。











































 京公网安备 11010802027423号
京公网安备 11010802027423号