当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioselective synthesis of fused ring heterocyclic derivatives of ketene aminals and their biological activities
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-07-01 , DOI: 10.1002/jhet.4014 Muhammad Yaqub 1 , Javeria Batool 1 , Khalid Mahmood 1 , Abida Ashraf 1, 2 , Ruqayia Perveen 1 , Zahid Shafiq 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-07-01 , DOI: 10.1002/jhet.4014 Muhammad Yaqub 1 , Javeria Batool 1 , Khalid Mahmood 1 , Abida Ashraf 1, 2 , Ruqayia Perveen 1 , Zahid Shafiq 1
Affiliation
|
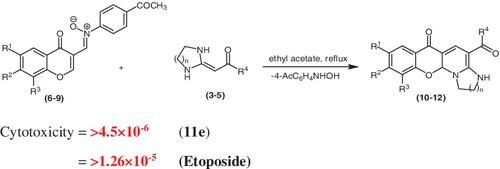
A regioselective and convenient methodology was developed to synthesize heterocyclic derivatives, bearing imidazole, piperidines, and azepines rings. The N ‐arylnitrones derived from 3‐formylchromones were selected to react with heterocyclic ketene aminal to furnish the structurally attractive and pharmacologically important fused ring heterocycles. The N ‐arylnitrone moiety of 3‐formylchromone was used to activate the formyl group for regioselective fused ring heterocycles synthesis, whereas, the effect of substituents at aryl functionality of nitrones were studied to improve the yield of target fused ring heterocyclic products. The synthesized compounds (10‐12) were evaluated for their in vitro cytotoxic and antifungal influences. In cytotoxic (brine shrimp lethality) assay, compound 11e was found to be active with LD50 = 4.1 × 10−6 μg/mL.
中文翻译:

烯酮缩醛的稠环杂环衍生物的区域选择性合成及其生物学活性
开发了区域选择性和方便的方法来合成带有咪唑,哌啶和氮杂环庚烷环的杂环衍生物。选择衍生自3-甲酰基色酮的N-芳基硝酮与杂环烯酮缩醛反应,以提供结构上有吸引力且在药理上重要的稠环杂环。3-甲酰基色酮的N-芳基亚硝基部分用于活化甲酰基以进行区域选择性稠合环杂环的合成,然而,研究了取代基对亚硝基芳基官能团的影响,以提高目标稠合环杂环产物的收率。合成的化合物(10-12)对它们的体外细胞毒性和抗真菌影响进行了评估。在细胞毒性(盐水虾致死率)测定中,发现化合物11e具有LD 50 = 4.1×10 -6μg / mL的活性。
更新日期:2020-08-08
中文翻译:

烯酮缩醛的稠环杂环衍生物的区域选择性合成及其生物学活性
开发了区域选择性和方便的方法来合成带有咪唑,哌啶和氮杂环庚烷环的杂环衍生物。选择衍生自3-甲酰基色酮的N-芳基硝酮与杂环烯酮缩醛反应,以提供结构上有吸引力且在药理上重要的稠环杂环。3-甲酰基色酮的N-芳基亚硝基部分用于活化甲酰基以进行区域选择性稠合环杂环的合成,然而,研究了取代基对亚硝基芳基官能团的影响,以提高目标稠合环杂环产物的收率。合成的化合物(10-12)对它们的体外细胞毒性和抗真菌影响进行了评估。在细胞毒性(盐水虾致死率)测定中,发现化合物11e具有LD 50 = 4.1×10 -6μg / mL的活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号