当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fe(III)‐Functionalized Magnetic Covalent Organic Frameworks for Fast Adsorption and Removal of Phenylbutazone in Aqueous Solution
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-07-02 , DOI: 10.1002/slct.202001671 Ruijuan Zheng 1 , Dan Feng 1 , Yan Xia 2
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-07-02 , DOI: 10.1002/slct.202001671 Ruijuan Zheng 1 , Dan Feng 1 , Yan Xia 2
Affiliation
|
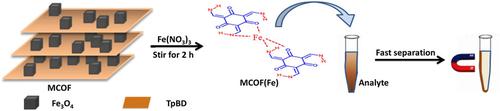
An Fe(III) functionalized magnetic covalent organic framework, named MCOF(Fe), was fast synthesized at ambient temperature for the removal of phenylbutazone in aqueous solution. The adsorption kinetics, isotherms, and thermodynamics were investigated to describe the adsorption process. The effects of adsorption factors and regeneration were studied as well. The adsorption mechanism may be explained with electrostatic interaction, π‐π interaction, and hydrogen bonding between phenylbutazone and the adsorbent. A pseudo‐second‐order rate equation described the adsorption kinetics mechanisms was revealed effectively, and phenylbutazone was removed in 2 h. The multi‐site Langmuir‐Freundlich model fitted adsorption process well, and the maximum adsorption capacity for phenylbutazone was 60.04 mg g−1 at 25 °C. Recyclability experiments indicated that MCOF(Fe) could be regenerated at least 3 times without significant loss of adsorption capacity. Finally, it can be suggested that MCOF(Fe) having excellent performance can be attractive for removal phenylbutazone from aqueous solution.
中文翻译:

Fe(III)-官能化磁性共价有机骨架,可快速吸附和去除水溶液中的苯基丁a
Fe(III)功能化的磁性共价有机骨架,称为MCOF(Fe),在室温下快速合成,用于去除水溶液中的苯基丁a。研究了吸附动力学,等温线和热力学来描述吸附过程。还研究了吸附因子和再生的影响。吸附机理可以用静电相互作用,π-π相互作用以及苯基丁the与吸附剂之间的氢键来解释。一个伪二级速率方程描述了吸附动力学机理,并在2 h内除去了苯基丁a。多位点Langmuir-Freundlich模型很好地拟合了吸附过程,苯基丁but的最大吸附容量为60.04 mg g -1在25°C下。可回收性实验表明,MCOF(Fe)可以再生至少3次,而不会显着降低吸附能力。最后,可以认为具有优异性能的MCOF(Fe)对于从水溶液中去除苯基丁a具有吸引力。
更新日期:2020-07-02
中文翻译:

Fe(III)-官能化磁性共价有机骨架,可快速吸附和去除水溶液中的苯基丁a
Fe(III)功能化的磁性共价有机骨架,称为MCOF(Fe),在室温下快速合成,用于去除水溶液中的苯基丁a。研究了吸附动力学,等温线和热力学来描述吸附过程。还研究了吸附因子和再生的影响。吸附机理可以用静电相互作用,π-π相互作用以及苯基丁the与吸附剂之间的氢键来解释。一个伪二级速率方程描述了吸附动力学机理,并在2 h内除去了苯基丁a。多位点Langmuir-Freundlich模型很好地拟合了吸附过程,苯基丁but的最大吸附容量为60.04 mg g -1在25°C下。可回收性实验表明,MCOF(Fe)可以再生至少3次,而不会显着降低吸附能力。最后,可以认为具有优异性能的MCOF(Fe)对于从水溶液中去除苯基丁a具有吸引力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号