当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reexamination of the Ergothioneine Biosynthetic Methyltransferase EgtD from Mycobacterium tuberculosis as a Protein Kinase Substrate.
ChemBioChem ( IF 2.6 ) Pub Date : 2020-07-02 , DOI: 10.1002/cbic.202000232 Alice Maurer 1 , Florian P Seebeck 1
ChemBioChem ( IF 2.6 ) Pub Date : 2020-07-02 , DOI: 10.1002/cbic.202000232 Alice Maurer 1 , Florian P Seebeck 1
Affiliation
|
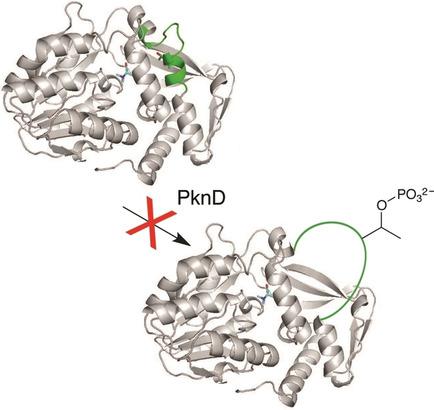
Conformer conundrum: The histidine methyltransferase EgtD produces N‐α‐trimethylhistidine as the first intermediate of ergothioneine biosynthesis in M. tuberculosis. This enzyme can be regulated by kinase‐mediated phosphorylation. However, in vitro characterization of this process suggests that folded EgtD is a poor substrate for protein kinase D (PknD).

中文翻译:

作为蛋白质激酶底物的结核分枝杆菌的麦角硫氨酸生物合成甲基转移酶EgtD的重新检查。
一致的难题:组氨酸甲基转移酶EgtD产生N -α-三甲基组氨酸作为结核分枝杆菌中麦角硫因生物合成的第一个中间体。该酶可以通过激酶介导的磷酸化来调节。但是,此过程的体外表征表明折叠的EgtD是蛋白激酶D(PknD)的较差底物。

更新日期:2020-07-02

中文翻译:

作为蛋白质激酶底物的结核分枝杆菌的麦角硫氨酸生物合成甲基转移酶EgtD的重新检查。
一致的难题:组氨酸甲基转移酶EgtD产生N -α-三甲基组氨酸作为结核分枝杆菌中麦角硫因生物合成的第一个中间体。该酶可以通过激酶介导的磷酸化来调节。但是,此过程的体外表征表明折叠的EgtD是蛋白激酶D(PknD)的较差底物。












































 京公网安备 11010802027423号
京公网安备 11010802027423号