当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recent Developments on the Synthesis of Various Sulfur‐Containing Heterocycles via [3+2]‐ and [4+2]‐Cycloaddition Reactions with Thiocarbonyls
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-07-02 , DOI: 10.1002/ajoc.202000238 Vandana Jaiswal 1 , Biplab Mondal 1 , Jaideep Saha 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-07-02 , DOI: 10.1002/ajoc.202000238 Vandana Jaiswal 1 , Biplab Mondal 1 , Jaideep Saha 1
Affiliation
|
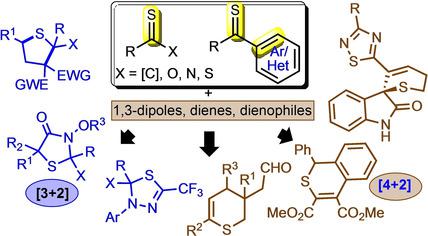
This minireview summarizes important collection of cycloaddition strategies that were used for the synthesis of sulfur‐containing five‐ and six‐membered heterocycles from thiocarbonyl‐based synthons. Among various stepwise or concerted methods available to prepare thioheterocycles, cycloaddition reactions of thiocarbonyls in particular present an important class of reaction owing to their ability to function as super dipolarophiles and/or superdienophiles. Design and development of various reactions in recent years with thiocarbonyls have certainly superseded the general purview on this reactive synthon that it often leads to side reaction and oligomerization. This article, apart from including the advances in the known reaction manifold of thiocarbonyls, also provide updates on new developments of cycloaddition chemistry, which includes donor‐acceptor (D−A) cyclopropanes and azaoxyallyl cation as 1,3‐dipolar reaction partners. This review should serve the purpose of an important guiding tool for organic and medicinal chemist to design and develop new class of cycloaddition or related transformations with thiocarbonyls for the preparation of novel heterocycles.
中文翻译:

通过[3 + 2]-和[4 + 2]-硫代羰基环加成反应合成各种含硫杂环的最新进展
这份小型综述总结了重要的环加成策略,这些策略用于从基于硫羰基的合成子合成含硫的五元和六元杂环。在可用于制备硫杂环的各种逐步或协同方法中,硫代羰基的环加成反应由于它们具有超双亲亲和/或超双亲亲和的功能而特别呈现出重要的一类反应。近年来,与硫代羰基的各种反应的设计和开发无疑已经取代了该反应性合成子的一般权限,因为它通常会导致副反应和低聚。除了介绍已知的硫代羰基反应歧管的进展外,本文还提供了环加成化学新进展的最新进展,其中包括供体受体(DA)环丙烷和氮杂烯丙基阳离子作为1,3-偶极反应伙伴。这项审查应为有机和药用化学家设计和开发新型的环加成或相关的硫代羰基相关转化制备新型杂环的重要指导工具。
更新日期:2020-07-02
中文翻译:

通过[3 + 2]-和[4 + 2]-硫代羰基环加成反应合成各种含硫杂环的最新进展
这份小型综述总结了重要的环加成策略,这些策略用于从基于硫羰基的合成子合成含硫的五元和六元杂环。在可用于制备硫杂环的各种逐步或协同方法中,硫代羰基的环加成反应由于它们具有超双亲亲和/或超双亲亲和的功能而特别呈现出重要的一类反应。近年来,与硫代羰基的各种反应的设计和开发无疑已经取代了该反应性合成子的一般权限,因为它通常会导致副反应和低聚。除了介绍已知的硫代羰基反应歧管的进展外,本文还提供了环加成化学新进展的最新进展,其中包括供体受体(DA)环丙烷和氮杂烯丙基阳离子作为1,3-偶极反应伙伴。这项审查应为有机和药用化学家设计和开发新型的环加成或相关的硫代羰基相关转化制备新型杂环的重要指导工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号