Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-07-02 , DOI: 10.1016/j.tetlet.2020.152182 Evans O. Onyango , Philip Z. Mannes , Alexandre Pletnev , Devin B. Granger , Qianxiang Ai , Chad Risko , John E. Anthony , Gordon W. Gribble
|
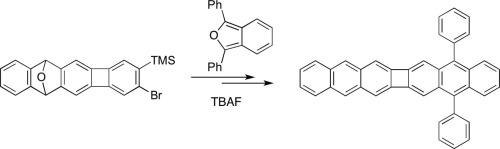
We describe the synthesis and electronic properties of the novel interrupted hexacene 5,16-diphenylcyclobuta[1,2-b:3,4-b′]dianthracene (1). The synthesis is rooted in a sequential twin Sonogashira coupling of 2,3-dibromo-9,10-dihydro-9,10-epoxyanthracene followed by a Vollhardt 2+2+2 cyclotrimerization to give o-bromoaryl(trimethylsilyl) 8, after selective bromination. A Diels-Alder cycloaddition of the aryne from 8 and 1,3-diphenylisobenzofuran gave 1 after deoxygenation. Photophysical studies suggest that the fused cyclobutane ring been the two anthracene rings allows through-conjugation, but does not allow 1 to behave as a conjugated acene. Attempts to synthesize the parent analogue, cyclobuta[1,2-b:3,4-b′]dianthracene (2) were not successful, but detailed calculations are reported for both 1 and 2.
中文翻译:

线性稠合蒽二聚体的合成及电子性质
我们描述了新型间断己烯5,16-二苯基环丁[1,2- b:3,4- b ']双蒽(1)的合成和电子性质。合成的基础是选择性的2,3-二溴-9,10-二氢-9,10-环氧蒽的连续双Sonogashira偶联,然后进行Vollhardt 2 + 2 + 2环三聚,得到邻溴代芳基(三甲基甲硅烷基)8。溴化。脱氧后,由8和1,3-二苯基异苯并呋喃对芳烃进行Diels-Alder环加成反应得到1。光物理研究表明,稠合的环丁烷环是两个蒽环允许直通共轭,但不允许1表现为共轭并苯。尝试合成母体类似物cyclobuta [1,2- b:3,4- b ']双蒽(2)失败,但详细的计算报告了1和2。











































 京公网安备 11010802027423号
京公网安备 11010802027423号