Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2020-07-02 , DOI: 10.1016/j.saa.2020.118669 Yue Xie 1 , Ping Tang 1 , Xinyue Xing 1 , Yao Zhao 2 , Shengqi Cao 1 , Shengde Liu 1 , Xiaoxu Lu 1 , Liyun Zhong 1
|
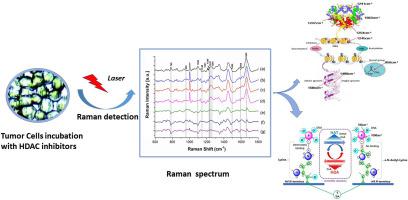
Though it has been demonstrated that Chidamide (CS055/HBI-8000), a novel benzamide class of histone deacetylase (HDAC) subtype-selectively inhibitor, reveals better anticancer effect in acute leukemia, but it remains unknown about the precise mechanism of Chidamide-induced acute leukemia cell apoptosis due to the lack of in situ molecular changes information. Based on Raman spectral analysis, we find that the action of Chidamide on Jurkat cell will lead to an addition of an acetyl group to a specific lysine residue at the end of histone amino acid, and greatly enhance the acetylation of histones H1, H2A, H2B, H3, and H4, and then destroy the electrostatic force between the alkaline terminal of the positive charged arginine side chain and the negative charged DNA of phosphate group, finally cause the depolymerization of DNA and histone octamer in chromatin nucleosome depolymerization and the relaxation of chromatin. Accordingly, the accumulation of reactive oxygen species (ROS) and the decreasing of mitochondrial membrane potential (MMP) are observed. For comparison, we also present the corresponding results of suberoylanilide hydroxamic acid (SAHA) and MS-275 inhibitors. The achieved results show that proliferation of Chidamide-treated Jurkat cells is low relative to MS-275 or SAHA, and the action of Chidamide or MS-275 on Jurkat cells lead to obvious increasing in histones H1, H2A, H2B, H3, and H4, whereas the action effect of SAHA is mainly observed in histones H1, H2A, H2B, H3 but weak in histone H4. Moreover, it is found that Chidamide-induced histone H3 acetylation in Jurkat cells is stronger than MS-275 and SAHA. Collectively, by Raman spectral analysis, we achieve the dynamic behavior of biochemical components, molecular conformation and morphological changes of HDAC inhibitors-treated Jurkat cells. Importantly, our research is the first to demonstrate that the action site of HDAC inhibitors on Jurkat cell is located in the DNA minor groove. Most importantly, the application of Raman spectrum in exploring in-situ molecular changes information, histone acetylation modification in epigenetics, drug action sites and cell cycle affected by HDAC inhibitors will supply new idea and reference for the design and modification of HDAC inhibitors.
中文翻译:

使用拉曼光谱法原位探索组蛋白脱乙酰基酶抑制剂Chidamide诱导白血病T淋巴细胞凋亡的分子变化。
尽管已经证明,新型的苯甲酰胺类组蛋白脱乙酰基酶(HDAC)亚型选择性抑制剂Chidamide(CS055 / HBI-8000)在急性白血病中显示出更好的抗癌作用,但是对于Chidamide诱导的确切机制尚不清楚急性白血病细胞凋亡由于缺乏原位分子变化信息。基于拉曼光谱分析,我们发现Chidamide对Jurkat细胞的作用将导致在组蛋白氨基酸末端的特定赖氨酸残基上添加一个乙酰基,并大大增强了组蛋白H1,H2A,H2B的乙酰化,H3和H4,然后破坏带正电荷的精氨酸侧链的碱性末端与带磷酸基团的带负电荷的DNA之间的静电力,最终在染色质核小体解聚和染色质松弛中引起DNA和组蛋白八聚体的解聚。因此,观察到活性氧(ROS)的积累和线粒体膜电位(MMP)的下降。为了进行比较,我们还介绍了辛二酰苯胺异羟肟酸(SAHA)和MS-275抑制剂的相应结果。获得的结果表明,相对于MS-275或SAHA,Chidamide处理的Jurkat细胞的增殖低,并且Chidamide或MS-275对Jurkat细胞的作用导致组蛋白H1,H2A,H2B,H3和H4明显增加。 ,而SAHA的作用效果主要在组蛋白H1,H2A,H2B,H3中观察到,而在组蛋白H4中则较弱。此外,发现Chidamide诱导的Jurkat细胞中的组蛋白H3乙酰化作用强于MS-275和SAHA。总的来说,通过拉曼光谱分析,我们获得了生化成分,分子构象和HDAC抑制剂处理的Jurkat细胞形态变化的动态行为。重要的是,我们的研究首次证明了HDAC抑制剂在Jurkat细胞上的作用位点位于DNA小沟中。最重要的是,拉曼光谱在探索原位分子变化信息,表观遗传学中的组蛋白乙酰化修饰,药物作用部位和受HDAC抑制剂影响的细胞周期方面的应用将为HDAC抑制剂的设计和修饰提供新的思路和参考。我们的研究首次证明HDAC抑制剂在Jurkat细胞上的作用位点位于DNA小沟中。最重要的是,拉曼光谱在探索原位分子变化信息,表观遗传学中的组蛋白乙酰化修饰,药物作用部位和受HDAC抑制剂影响的细胞周期方面的应用将为HDAC抑制剂的设计和修饰提供新的思路和参考。我们的研究首次证明HDAC抑制剂在Jurkat细胞上的作用位点位于DNA小沟中。最重要的是,拉曼光谱在探索原位分子变化信息,表观遗传学中的组蛋白乙酰化修饰,药物作用部位和受HDAC抑制剂影响的细胞周期方面的应用将为HDAC抑制剂的设计和修饰提供新的思路和参考。











































 京公网安备 11010802027423号
京公网安备 11010802027423号