Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2020-07-02 , DOI: 10.1016/j.saa.2020.118681 Tao Lu 1 , Juncheng Lei 1 , Qian Gou 1 , Gang Feng 1
|
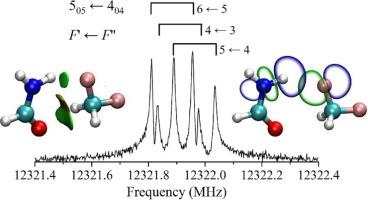
The pure rotational spectrum of the complex of difluoromethane with formamide was investigated by means of microwave spectroscopy supplemented with theoretical calculations. The hyperfine structure arising from the 14N nuclear quadrupole coupling effect was completely resolved. The most stable isomer that displays the Cs symmetry with the ∠HCH angle of difluoromethane being bisected by the ab-plane of formamide was detected. The two moieties in the detected isomer are connected via one N–H⋯F and one bifurcated CH2⋯O weak hydrogen bonds confirmed by the non-covalent interaction plot and natural bond orbital analyses. The distances of the NH⋯F and CH2⋯O interactions were determined to be 2.140(14) Å and 2.749(14) Å, respectively. The N
H⋯F bond angle was determined to be 150.7°. Symmetry-adapted perturbation theory analysis indicates that the electrostatic component is the largest contributor to the total attractive interaction energy of the difluoromethane⋯formamide complex.
中文翻译:

卤代烷和酰胺之间的氢键弱:二氟甲烷⋯甲酰胺配合物的微波光谱和理论研究。
通过微波光谱法和理论计算,研究了二氟甲烷与甲酰胺的配合物的纯旋转光谱。完全解析了由14 N核四极耦合效应产生的超精细结构。最稳定的异构体,显示Ç小号与二氟甲烷的∠HCH角对称性由等分的AB检测甲酰胺-平面。通过非共价相互作用图和自然键轨道分析证实,检测到的异构体中的两个部分通过一个N-H⋯F和一个分叉的CH 2 = O弱氢键相连。N H⋯F和CH 2的距离⋯O相互作用确定为2.140(14)Å和2.749(14)Å。N
H⋯F键角确定为150.7°。适应对称性的扰动理论分析表明,静电成分是二氟甲烷⋯甲酰胺络合物总吸引相互作用能的最大贡献者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号