Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2020-07-02 , DOI: 10.1016/j.molliq.2020.113720 Dorota Kołodyńska , Katarzyna Araucz
|
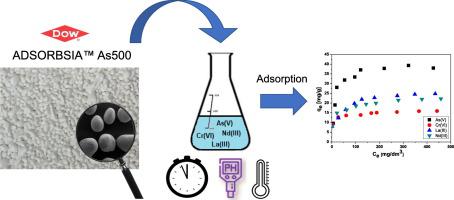
A new commercial sorbent ADSORBSIA™ As500 was used for As(V) and Cr(VI) removal as well as La(III) and Nd(III) recovery from aqueous solutions. The physicochemical properties were characterized by nitrogen adsorption/desorption analyses, scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), and X-ray diffraction (XRD). The adsorption kinetics of ADSORBSIA™ As500 was analysed using the reaction and diffusion base models such as the pseudo-first order, pseudo-second order, intraparticle diffusion, and Dumwald-Wagner. The effect of different pH values, dose, initial concentrations and the type of metal ions on the adsorption capacity was analysed. As for adsorption data, the Langmuir, Freundlich and Temkin models were used. Desorption of adsorbed metal ions from ADSORBSIA™ As500 was not studied. The sorption equilibrium was established within 60 min. The maximum amount of metals (qe) adsorbed at equilibrium was 37.98, 15.91, 24.77 and 22.23 mg/g for As(V), Cr(VI), La(III) and Nd(III), respectively, which can be arranged in the following selectivity order: As(V) > La(III) > Nd(III) > Cr(VI). The sorption capacities were found to be pH, time, concentration and sorbent dose dependent. The equilibrium data were well described by the Langmuir adsorption isotherm, but the kinetic data were fitted by the pseudo-second order model.
中文翻译:

用于去除As(V)和Cr(VI)以及回收La(III)和Nd(III)的新型二氧化钛吸附剂
新型商业吸附剂ADSORBSIA™As500用于去除水中的As(V)和Cr(VI)以及回收La(III)和Nd(III)。通过氮吸附/解吸分析,扫描电子显微镜(SEM),傅立叶变换红外光谱(FTIR)和X射线衍射(XRD)表征其理化性质。使用反应和扩散基本模型(例如拟一级,拟二级,颗粒内扩散和Dumwald-Wagner)分析ADSORBSIA™As500的吸附动力学。分析了不同pH值,剂量,初始浓度和金属离子类型对吸附容量的影响。至于吸附数据,使用Langmuir,Freundlich和Temkin模型。未研究从ADSORBSIA™As500解吸吸附的金属离子。在60分钟内建立了吸附平衡。最大金属量(qe)吸附的平衡态e)对于As(V),Cr(VI),La(III)和Nd(III)分别为37.98、15.91、24.77和22.23 mg / g,可以按以下选择性顺序排列: (V)> La(III)> Nd(III)> Cr(VI)。发现吸附能力取决于pH,时间,浓度和吸附剂剂量。朗格缪尔吸附等温线很好地描述了平衡数据,而动力学数据通过拟二阶模型拟合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号