当前位置:
X-MOL 学术
›
Inorg. Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly selective and potent anti-cancer agents based on 2,9-substituted-1,10-phenanthroline derivatives
Inorganic Chemistry Communications ( IF 4.4 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.inoche.2020.108085 Sourav De , S.K. Ashok Kumar
Inorganic Chemistry Communications ( IF 4.4 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.inoche.2020.108085 Sourav De , S.K. Ashok Kumar
|
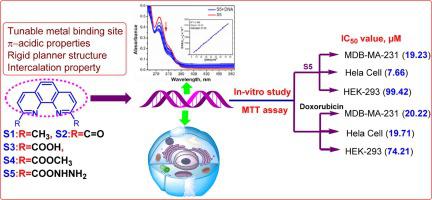
Abstract A study concerning on in-vitro anticancer evaluation of structurally tuned 1,10-phenanthroline at 2,9 positions with different functional groups such as –CH3 (S1), >C O (S2), –COOH (S3), –COOCH3 (S4) and –CONHNH2 (S5) were described. The solubility data revealed that all ligands were completely soluble in dimethyl sulphoxide (DMSO) and moderately soluble in water. The photo-physical properties of these ligands revealed that a common absorption peak appeared in the region of 270–300 nm and emission spectra in the region of 330–510 nm with a large Stokes shift of 85 nm. The binding constant of ligands (S1-S5) with calf-thymus deoxyribonucleic acid (CT-DNA) and bovine serum albumin (BSA) were found to be 105 M−1 and 104 M−1 respectively. The fluorescence quenching of ethidium bromide (EtBr) from DNA upon addition of ligand was confirmed from binding affinity values KSV (104 M−1) and Kapp (106 M−1). The mode of interaction of ligand with DNA is either by intercalation or groove binding this is further supported by viscosity and in-silico studies. The gel electrophoresis studies exhibited that S4 and S5 have cleaved plasmid DNA completely within 60 min while rest of the ligands took more than 60 min. The cytotoxicity study of these ligands (S1-S5) were conducted with two different cancer cell lines (MDA-MB-231 and HeLa) and their performance were compared with normal HEK-293 cells. The study revealed that ligands S5 and S4 were found to be least inhibitory concentration (IC50) and high selectivity factor values of 7.66 µM/12.97 and 13.35 µM/6.19 with respect to HeLa and MDA-MB-231 cell lines while ligands S1-S3 showed high IC50 values compare to doxorubicin. However, both S5 and S4 ligands have been displayed higher cytotoxicity effect than doxorubicin and least effect on normal cell HEK-293.
中文翻译:

基于 2,9-取代-1,10-菲咯啉衍生物的高选择性和强效抗癌剂
摘要 一项关于在 2,9 位结构调整的 1,10-菲咯啉具有不同官能团如 –CH3 (S1)、>CO (S2)、–COOH (S3)、–COOCH3 ( S4) 和 –CONHNH2 (S5) 进行了描述。溶解度数据显示所有配体完全溶于二甲基亚砜(DMSO),中等溶于水。这些配体的光物理性质表明,共同的吸收峰出现在 270-300 nm 区域,发射光谱出现在 330-510 nm 区域,斯托克斯位移大为 85 nm。发现配体 (S1-S5) 与小牛胸腺脱氧核糖核酸 (CT-DNA) 和牛血清白蛋白 (BSA) 的结合常数分别为 105 M-1 和 104 M-1。从结合亲和力值 KSV (104 M-1) 和 Kapp (106 M-1) 证实了加入配体后 DNA 中溴化乙锭 (EtBr) 的荧光猝灭。配体与 DNA 的相互作用模式是通过嵌入或凹槽结合,这得到了粘度和计算机内研究的进一步支持。凝胶电泳研究表明,S4 和 S5 已在 60 分钟内完全切割质粒 DNA,而其余配体则需要 60 多分钟。这些配体 (S1-S5) 的细胞毒性研究是用两种不同的癌细胞系 (MDA-MB-231 和 HeLa) 进行的,并将它们的性能与正常 HEK-293 细胞进行比较。研究表明,发现配体 S5 和 S4 具有最低抑制浓度 (IC50) 和高选择性因子值,分别为 7.66 µM/12.97 和 13.35 µM/6。19 关于 HeLa 和 MDA-MB-231 细胞系,而配体 S1-S3 与阿霉素相比显示出高 IC50 值。然而,S5 和 S4 配体均显示出比阿霉素更高的细胞毒性作用,而对正常细胞 HEK-293 的影响最小。
更新日期:2020-09-01
中文翻译:

基于 2,9-取代-1,10-菲咯啉衍生物的高选择性和强效抗癌剂
摘要 一项关于在 2,9 位结构调整的 1,10-菲咯啉具有不同官能团如 –CH3 (S1)、>CO (S2)、–COOH (S3)、–COOCH3 ( S4) 和 –CONHNH2 (S5) 进行了描述。溶解度数据显示所有配体完全溶于二甲基亚砜(DMSO),中等溶于水。这些配体的光物理性质表明,共同的吸收峰出现在 270-300 nm 区域,发射光谱出现在 330-510 nm 区域,斯托克斯位移大为 85 nm。发现配体 (S1-S5) 与小牛胸腺脱氧核糖核酸 (CT-DNA) 和牛血清白蛋白 (BSA) 的结合常数分别为 105 M-1 和 104 M-1。从结合亲和力值 KSV (104 M-1) 和 Kapp (106 M-1) 证实了加入配体后 DNA 中溴化乙锭 (EtBr) 的荧光猝灭。配体与 DNA 的相互作用模式是通过嵌入或凹槽结合,这得到了粘度和计算机内研究的进一步支持。凝胶电泳研究表明,S4 和 S5 已在 60 分钟内完全切割质粒 DNA,而其余配体则需要 60 多分钟。这些配体 (S1-S5) 的细胞毒性研究是用两种不同的癌细胞系 (MDA-MB-231 和 HeLa) 进行的,并将它们的性能与正常 HEK-293 细胞进行比较。研究表明,发现配体 S5 和 S4 具有最低抑制浓度 (IC50) 和高选择性因子值,分别为 7.66 µM/12.97 和 13.35 µM/6。19 关于 HeLa 和 MDA-MB-231 细胞系,而配体 S1-S3 与阿霉素相比显示出高 IC50 值。然而,S5 和 S4 配体均显示出比阿霉素更高的细胞毒性作用,而对正常细胞 HEK-293 的影响最小。











































 京公网安备 11010802027423号
京公网安备 11010802027423号