European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-07-02 , DOI: 10.1016/j.ejmech.2020.112549 Xiaomei Chen 1 , Li Ding 2 , Yuan Tao 2 , Christophe Pannecouque 3 , Erik De Clercq 3 , Chunlin Zhuang 2 , Fen-Er Chen 4
|
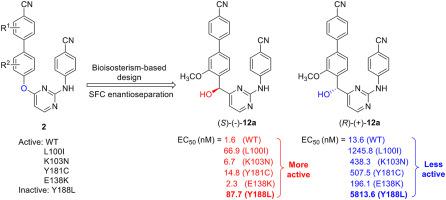
The single enantiomers of seven hydroxyl-substituted biphenyl-diarylpyrimidines were designed and synthesized by a bioisosterism strategy as novel HIV-1 non-nucleoside reverse transcriptase inhibitors (NNRTIs). The cellular and enzymatic assays indicated that the novel obtained compounds had significant activities and relatively low cytotoxicity. The supercritical fluid chromatography (SFC) enantioseparations of the racemic compounds and the enantiomeric profiling resulted that the (S) forms were generally more potent than the (R) counterparts. Among all the chiral derivatives, (S)-(-)-12a showed the best potency with the antiviral activities against wild-type (WT) and single mutant strains (L100I, K103N, Y181C, E138K; especially Y188L), and RT enzyme in the low nanomolar concentration range. Toward double mutant virus strains (F227L+V106A, RES056), (S)-(-)-12a possessed submicromolar antiviral activities. In addition, (S)-(-)-12a showed a high cell-based selectivity index (SI WT = 5822) and no apparent toxicity was observed in the in vivo acute toxicity assay and electrocardiogram. The molecular docking studies predicted the binding modes of the compounds with RT and explained the activity differences for the enantiomers. Although the rat pharmacokinetic assay indicated a poor oral metabolism of the hydroxyl compound, the promising antiviral activity of the chiral hydroxyl-substituted biphenyl-diarylpyrimidines provided valuable lead compounds for further anti-HIV drug design.
中文翻译:

基于手性羟基取代的联苯-二芳基嘧啶非核苷HIV-1逆转录酶抑制剂的基于生物立体异构的设计和对映体分析。
七个羟基取代的联苯-二芳基嘧啶的单一对映体是通过生物立体异构策略设计和合成的,作为新型HIV-1非核苷逆转录酶抑制剂(NNRTIs)。细胞和酶促测定表明,新获得的化合物具有显着的活性和相对较低的细胞毒性。外消旋化合物的超临界流体色谱(SFC)对映体分离和对映体分析表明,(S)形式通常比(R)对应物更有效。在所有手性衍生物中,(S)-(-)- 12a在低纳摩尔浓度范围内,对野生型(WT)和单个突变菌株(L100I,K103N,Y181C,E138K;特别是Y188L)和RT酶具有最佳的抗病毒活性。对双重突变病毒株(F227L + V106A,RES056),(S)-(-)- 12a具有亚微摩尔抗病毒活性。此外,(S)-(-)- 12a显示出高的基于细胞的选择性指数(SI WT= 5822),并且在体内急性毒性试验和心电图中未观察到明显的毒性。分子对接研究预测了化合物与RT的结合模式,并解释了对映体的活性差异。尽管大鼠药代动力学分析表明羟基化合物的口服代谢不良,但手性羟基取代的联苯-二芳基嘧啶的有希望的抗病毒活性为进一步的抗HIV药物设计提供了有价值的先导化合物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号