Current Biology ( IF 8.1 ) Pub Date : 2020-07-02 , DOI: 10.1016/j.cub.2020.05.090 J Sebastián Gómez-Cavazos 1 , Kian-Yong Lee 2 , Pablo Lara-González 2 , Yanchi Li 2 , Arshad Desai 3 , Andrew K Shiau 4 , Karen Oegema 3
|
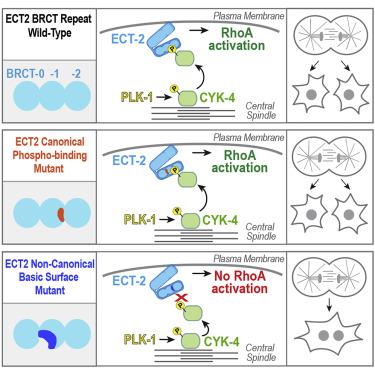
Cytokinesis partitions the cell contents to complete mitosis. During cytokinesis, polo-like kinase 1 (PLK1) activates the small GTPase RhoA to assemble a contractile actomyosin ring. PLK1 is proposed to pattern RhoA activation by creating a docking site on the central spindle that concentrates the RhoA guanine nucleotide exchange factor ECT2. However, ECT2 targeting to the central spindle is dispensable for cytokinesis, indicating that how PLK1 controls RhoA activation remains unresolved. To address this question, we employed an unbiased approach targeting ∼100 predicted PLK1 sites in two RhoA regulators: ECT2 and the centralspindlin complex, composed of CYK4 and kinesin-6. This comprehensive approach suggested that the only functionally critical PLK1 target sites are in a single cluster in the CYK4 N terminus. Phosphorylation of this cluster promoted direct interaction of CYK4 with the BRCT repeat module of ECT2. However, mutational analysis in vitro and in vivo led to the surprising finding that the interaction was independent of the conserved “canonical” residues in ECT2’s BRCT repeat module that, based on structurally characterized BRCT-phosphopeptide interactions, were presumed critical for binding. Instead, we show that the ECT2 BRCT module binds phosphorylated CYK4 via a distinct conserved basic surface. Basic surface mutations mimic the effects on cytokinesis of loss of CYK4 cluster phosphorylation or inhibition of PLK1 activity. Together with evidence for ECT2 autoinhibition limiting interaction with CYK4 in the cytoplasm, these results suggest that a spatial gradient of phosphorylated CYK4 around the central spindle patterns RhoA activation by interacting with ECT2 on the adjacent plasma membrane.
中文翻译:

细胞分裂过程中 RhoA 激活的基础是非经典 BRCT-磷酸肽识别机制。
胞质分裂分配细胞内容物以完成有丝分裂。在胞质分裂过程中,polo 样激酶 1 (PLK1) 激活小 GTP 酶 RhoA 以组装收缩性肌动球蛋白环。 PLK1 被提议通过在集中 RhoA 鸟嘌呤核苷酸交换因子 ECT2 的中央纺锤体上创建对接位点来模拟 RhoA 激活。然而,靶向中央纺锤体的 ECT2 对于胞质分裂来说是可有可无的,这表明 PLK1 如何控制 RhoA 激活仍未解决。为了解决这个问题,我们采用了一种公正的方法,针对两个 RhoA 调节因子中约 100 个预测的 PLK1 位点:ECT2 和由 CYK4 和驱动蛋白 6 组成的中枢纺锤蛋白复合体。这种综合方法表明,唯一功能关键的 PLK1 靶位点位于 CYK4 N 末端的单个簇中。该簇的磷酸化促进了 CYK4 与 ECT2 的 BRCT 重复模块的直接相互作用。然而,体外和体内突变分析得出了令人惊讶的发现,即相互作用独立于 ECT2 BRCT 重复模块中保守的“规范”残基,根据结构特征的 BRCT-磷酸肽相互作用,推测该残基对于结合至关重要。相反,我们表明 ECT2 BRCT 模块通过独特的保守基本表面结合磷酸化 CYK4。基本表面突变模拟 CYK4 簇磷酸化丧失或 PLK1 活性抑制对胞质分裂的影响。结合 ECT2 自抑制限制与细胞质中 CYK4 相互作用的证据,这些结果表明,中央纺锤体周围磷酸化 CYK4 的空间梯度通过与相邻质膜上的 ECT2 相互作用而激活 RhoA。











































 京公网安备 11010802027423号
京公网安备 11010802027423号