Current Biology ( IF 8.1 ) Pub Date : 2020-07-02 , DOI: 10.1016/j.cub.2020.05.091 João Carlos Gonçalves 1 , Sebastian Quintremil 2 , Julie Yi 2 , Richard B Vallee 2
|
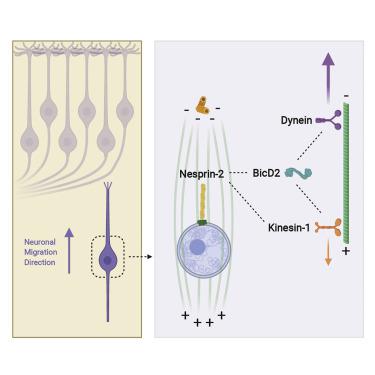
Vertebrate brain development depends on a complex program of cell proliferation and migration. Post-mitotic neuronal migration in the developing cerebral cortex involves Nesprin-2, which recruits cytoplasmic dynein, kinesin, and actin to the nuclear envelope (NE) in other cell types. However, the relative importance of these interactions in neurons has remained poorly understood. To address these issues, we performed in utero electroporation into the developing rat brain to interfere with Nesprin-2 function. We find that an ∼100-kDa “mini” form of the ∼800-kDa Nesprin-2 protein, which binds dynein and kinesin, is sufficient, remarkably, to support neuronal migration. In contrast to dynein’s role in forward nuclear migration in these cells, we find that kinesin-1 inhibition accelerates neuronal migration, suggesting a novel role for the opposite-directed motor proteins in regulating migration velocity. In contrast to studies in fibroblasts, the actin-binding domain of Nesprin-2 was dispensable for neuronal migration. We find further that, surprisingly, the motor proteins interact with Nesprin-2 through the dynein/kinesin “adaptor” BicD2, both in neurons and in non-mitotic fibroblasts. Furthermore, mutation of the Nesprin-2 LEWD sequence, implicated in nuclear envelope kinesin recruitment in other systems, interferes with BicD2 binding. Although disruption of the Nesprin-2/BicD2 interaction severely inhibited nuclear movement, centrosome advance proceeded unimpeded, supporting an independent mechanism for centrosome advance. Our data together implicate Nesprin-2 as a novel and fundamentally important form of BicD2 cargo and help explain BicD2’s role in neuronal migration and human disease.
中文翻译:

Nesprin-2 将 BicD2 募集至核膜可控制动力蛋白/驱动蛋白介导的体内神经元迁移。
脊椎动物大脑的发育取决于细胞增殖和迁移的复杂程序。发育中的大脑皮层中的有丝分裂后神经元迁移涉及 Nesprin-2,它将细胞质动力蛋白、驱动蛋白和肌动蛋白募集到其他细胞类型的核膜 (NE)。然而,人们对神经元中这些相互作用的相对重要性仍然知之甚少。为了解决这些问题,我们在子宫内对发育中的大鼠大脑进行电穿孔,以干扰 Nesprin-2 功能。我们发现~100-kDa“迷你”形式的~800-kDa Nesprin-2蛋白结合动力蛋白和驱动蛋白,足以支持神经元迁移。与动力蛋白在这些细胞中向前核迁移中的作用相反,我们发现驱动蛋白-1 抑制加速了神经元迁移,这表明相反方向的运动蛋白在调节迁移速度方面具有新的作用。与成纤维细胞的研究相反,Nesprin-2 的肌动蛋白结合域对于神经元迁移来说是可有可无的。我们进一步发现,令人惊讶的是,在神经元和非有丝分裂成纤维细胞中,运动蛋白通过动力蛋白/驱动蛋白“适配器”BicD2 与 Nesprin-2 相互作用。此外,Nesprin-2 LEWD 序列的突变与其他系统中核膜驱动蛋白的募集有关,会干扰 BicD2 结合。尽管 Nesprin-2/BicD2 相互作用的破坏严重抑制了核运动,但中心体前进仍不受阻碍,支持中心体前进的独立机制。我们的数据表明 Nesprin-2 是 BicD2 货物的一种新型且极其重要的形式,并有助于解释 BicD2 在神经元迁移和人类疾病中的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号