Biochimie ( IF 3.3 ) Pub Date : 2020-07-02 , DOI: 10.1016/j.biochi.2020.06.007 Anna S Dotsenko 1 , Gleb S Dotsenko 2 , Aleksandra M Rozhkova 1 , Ivan N Zorov 3 , Arkady P Sinitsyn 3
|
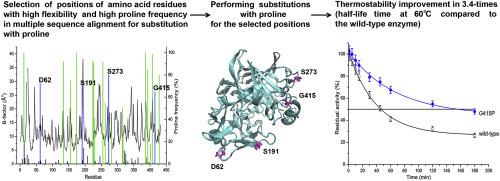
Thermostability is a fundamental characteristic of enzymes that is of high importance for industrial implementation of enzymatic catalysis. Cellobiohydrolases are enzymes capable to hydrolyze the most abundant natural polysaccharide – cellulose. These enzymes are widely applied in industry for processing of cellulose containing materials. However, structural and functional engineering of cellobiohydrolases for improving their properties is a challenging task. In this study, the thermostability of Penicillium verruculosum Cel7A cellobiohydrolase was increased through rational design of substitutions with proline. The stabilizing substitution G415P resulted in 3.4-fold increase in half-life time at 60 °C compared to wild-type enzyme. Molecular dynamics simulations indicated a clear effect of the stabilizing substitution G415P and the destabilizing substitutions D62P, S191P, and S273P on the stability of the enzyme tertiary structure. The stabilizing substitution G415P decreased flexibility of the lateral sides of the enzyme active site tunnel, while the considered destabilizing substitutions increased their flexibility.
中文翻译:

合理设计和结构见解,可改善Verenculiumum vericulumum Cel7A纤维二糖水解酶的热稳定性。
热稳定性是酶的基本特征,其对于工业实施酶催化非常重要。纤维二糖水解酶是能够水解最丰富的天然多糖纤维素的酶。这些酶在工业上广泛用于处理含纤维素的材料。然而,纤维二糖水解酶的结构和功能工程以改善其性能是一项艰巨的任务。在这项研究中,绿青霉的热稳定性通过合理设计脯氨酸取代,Cel7A纤维二糖水解酶增加。与野生型酶相比,稳定取代G415P在60°C下的半衰期增加了3.4倍。分子动力学模拟表明,稳定取代基G415P和不稳定取代基D62P,S191P和S273P对酶三级结构的稳定性有明显影响。稳定取代基G415P降低了酶活性位点通道侧面的柔韧性,而认为的不稳定取代基则增加了其柔韧性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号