当前位置:
X-MOL 学术
›
Appl. Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Dissociation of Benzoic Acid on Carbonate Surfaces: A Density Functional Theory Perspective
Applied Surface Science ( IF 6.3 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.apsusc.2020.147103 Filipe Camargo Dalmatti Alves Lima , Raphael da Silva Alvim , Caetano Rodrigues Miranda
Applied Surface Science ( IF 6.3 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.apsusc.2020.147103 Filipe Camargo Dalmatti Alves Lima , Raphael da Silva Alvim , Caetano Rodrigues Miranda
|
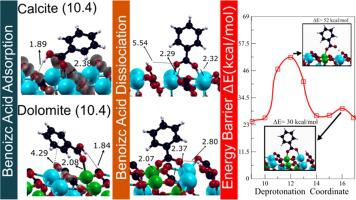
Abstract The adsorption of benzoic acid (BA) and the consequent benzoate (B) formation are fundamental processes responsible for wettability alteration on carbonate surfaces in the carbonate-oil interface. However, mixed wettability due to the occurrence of both Ca2+ and Mg2+ ions leads to current not well-understood changes in chemical properties related to BA and B formation. Therefore, we investigated by Density-Functional Theory a dissociative adsorption mechanism through different parallel and perpendicular conformations of BA and B on the calcite and dolomite ( 10 1 - 4 ) surfaces. In particular, BA is stably adsorbed on both carbonate surfaces main due to charge distribution between the carboxyl group and the surface cations. Furthermore, we find that the hydroxyl group tends to be available on dolomite rather than on calcite, being experimentally supported as the source for the possible BA selective adsorption. The calculated dissociation energy barrier also shows that dolomite surface displays the ideal condition just for a possible kinetic dependence upon the presence of water molecules. Therefore, we concluded that the mixture of calcite and dolomite portions in the reservoir surface could indeed work together upon the B formation. Accordingly, our results provide fundamental insights for the dissociation of acid oil components on mixed carbonate surfaces.
中文翻译:

碳酸盐表面苯甲酸的选择性离解:密度泛函理论视角
摘要 苯甲酸 (BA) 的吸附和随之而来的苯甲酸酯 (B) 的形成是导致碳酸盐-油界面中碳酸盐表面润湿性改变的基本过程。然而,由于 Ca2+ 和 Mg2+ 离子的出现而导致的混合润湿性导致目前尚不清楚与 BA 和 B 形成相关的化学性质的变化。因此,我们通过密度泛函理论研究了通过 BA 和 B 在方解石和白云石 ( 10 1 - 4 ) 表面上不同平行和垂直构象的解离吸附机制。特别是,由于羧基和表面阳离子之间的电荷分布,BA 稳定地吸附在两个碳酸酯表面上。此外,我们发现羟基倾向于在白云石而不是方解石上可用,被实验支持作为可能的 BA 选择性吸附的来源。计算出的解离能垒还表明,白云石表面显示出理想条件,只是因为可能存在对水分子的动力学依赖性。因此,我们得出结论,储层表面方解石和白云石部分的混合物确实可以共同作用于 B 地层。因此,我们的结果为混合碳酸盐表面上酸性油组分的解离提供了基本见解。我们得出结论,储层表面方解石和白云石部分的混合物确实可以共同作用于 B 地层。因此,我们的结果为混合碳酸盐表面上酸性油组分的解离提供了基本见解。我们得出结论,储层表面方解石和白云石部分的混合物确实可以共同作用于 B 地层。因此,我们的结果为混合碳酸盐表面上酸性油组分的解离提供了基本见解。
更新日期:2020-11-01
中文翻译:

碳酸盐表面苯甲酸的选择性离解:密度泛函理论视角
摘要 苯甲酸 (BA) 的吸附和随之而来的苯甲酸酯 (B) 的形成是导致碳酸盐-油界面中碳酸盐表面润湿性改变的基本过程。然而,由于 Ca2+ 和 Mg2+ 离子的出现而导致的混合润湿性导致目前尚不清楚与 BA 和 B 形成相关的化学性质的变化。因此,我们通过密度泛函理论研究了通过 BA 和 B 在方解石和白云石 ( 10 1 - 4 ) 表面上不同平行和垂直构象的解离吸附机制。特别是,由于羧基和表面阳离子之间的电荷分布,BA 稳定地吸附在两个碳酸酯表面上。此外,我们发现羟基倾向于在白云石而不是方解石上可用,被实验支持作为可能的 BA 选择性吸附的来源。计算出的解离能垒还表明,白云石表面显示出理想条件,只是因为可能存在对水分子的动力学依赖性。因此,我们得出结论,储层表面方解石和白云石部分的混合物确实可以共同作用于 B 地层。因此,我们的结果为混合碳酸盐表面上酸性油组分的解离提供了基本见解。我们得出结论,储层表面方解石和白云石部分的混合物确实可以共同作用于 B 地层。因此,我们的结果为混合碳酸盐表面上酸性油组分的解离提供了基本见解。我们得出结论,储层表面方解石和白云石部分的混合物确实可以共同作用于 B 地层。因此,我们的结果为混合碳酸盐表面上酸性油组分的解离提供了基本见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号