当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NaCl-promoted phase transition and glycosidic bond cleavage under microwave heating for energy-efficient biorefinery of rice starch
Green Chemistry ( IF 9.3 ) Pub Date : 2020-07-01 , DOI: 10.1039/d0gc01761b Iris K. M. Yu 1, 2, 3, 4, 5 , Jiajun Fan 1, 2, 3, 4, 5 , Vitaliy L. Budarin 1, 2, 3, 4, 5 , Florent P. Bouxin 1, 2, 3, 4, 5 , James H. Clark 1, 2, 3, 4, 5 , Daniel C. W. Tsang 6, 7, 8, 9
Green Chemistry ( IF 9.3 ) Pub Date : 2020-07-01 , DOI: 10.1039/d0gc01761b Iris K. M. Yu 1, 2, 3, 4, 5 , Jiajun Fan 1, 2, 3, 4, 5 , Vitaliy L. Budarin 1, 2, 3, 4, 5 , Florent P. Bouxin 1, 2, 3, 4, 5 , James H. Clark 1, 2, 3, 4, 5 , Daniel C. W. Tsang 6, 7, 8, 9
Affiliation
|
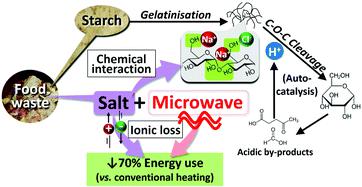
Sustainable utilisation of food waste in biorefineries is imperative to actualising a circular economy and alleviating the massive environmental burden. An efficient biorefinery system design necessitates comprehensive understanding of the impurity effects such as salt. This study scrutinised the hydrolysis of rice starch, a major component in food waste, in NaCl(aq) solution under robust microwave heating, without adding catalysts and organic solvents to pursue green chemistry. The NaCl-microwave synergy was revealed via an innovative approach, by tracing and assimilating the data of microwave real-time power output, in-vessel pressure, and temperature gradient across the reactant matrix. The use of very diluted NaCl(aq) (0.05 wt%) increased the total product yields from 23.6 to 37.4 mol%, with tetra/tri/disaccharides and glucose being the major products after reaction at 200 °C for 15 min. The highest product yields of 65.9 mol% could be obtained in 1 wt% NaCl(aq). Fluctuation in the temperature measurement during ramping signified the NaCl-promoted gelatinisation, in which the reactant mixture exhibited rapid changes in viscosity. The 13C solid-state nuclear magnetic resonance spectroscopy suggested the possible selective interactions between NaCl and the free hydroxyl groups at C6 and C2,3,5 positions, whereas α(1 → 4) linkages were less assessable due to steric hindrance. The hydrolytic depolymerisation of starch was auto-catalysed by acid species generated in situ at the temperature holding stage. This step was accelerated by the presence of NaCl that might interact with and weaken the glycosidic bonds. From an energy perspective, the use of microwaves in place of conventional heating reduced the energy consumption by 70% to achieve the same products profile, as NaCl enabled superior dielectric heating via ionic loss. This study offers new insights into the NaCl-polysaccharide interactions and unique NaCl functions under microwave conditions, which are necessary fundamentals to develop green and energy-efficient biorefinery systems.
中文翻译:

微波加热下NaCl促进的相变和糖苷键裂解,用于大米淀粉的高效生物精制
生物炼油厂中食物垃圾的可持续利用对于实现循环经济和减轻巨大的环境负担至关重要。高效的生物精炼系统设计需要全面了解诸如盐之类的杂质影响。这项研究详细研究了在强大的微波加热下,在不添加催化剂和有机溶剂以追求绿色化学的情况下,米粉在NaCl (aq)溶液中的水解,它是食品废料中的主要成分。的NaCl的微波协同揭示通过一种创新的方法,通过跟踪和整个反应物基质同化微波实时功率输出,在血管的压力,和温度梯度的数据。使用极稀释的NaCl (水溶液)(0.05wt%)将总产物收率从23.6mol%增加到37.4mol%,其中四/三/二糖和葡萄糖是在200℃反应15分钟后的主要产物。在1wt%的NaCl (水溶液)中可获得最高的65.9mol%的产物产率。升温期间温度测量值的波动表示NaCl促进了糊化,其中反应混合物的粘度迅速变化。在13下为固体状态的核磁共振光谱表明NaCl和在C6和C2,3,5位置的游离羟基基团之间的可能的相互作用的选择性,而α(1→4)键不太课税由于空间位阻。淀粉的水解解聚由原位产生的酸物质自动催化在保温阶段。可能与糖苷键相互作用并减弱糖苷键的氯化钠的存在促进了这一步骤。从能源的角度来看,使用微波代替常规加热可将能耗降低70%,以实现相同的产品特性,因为氯化钠可通过离子损失实现出色的介电加热。这项研究为微波条件下的氯化钠-多糖相互作用和独特的氯化钠功能提供了新的见解,这是开发绿色和节能生物精炼系统的必要基础。
更新日期:2020-07-06
中文翻译:

微波加热下NaCl促进的相变和糖苷键裂解,用于大米淀粉的高效生物精制
生物炼油厂中食物垃圾的可持续利用对于实现循环经济和减轻巨大的环境负担至关重要。高效的生物精炼系统设计需要全面了解诸如盐之类的杂质影响。这项研究详细研究了在强大的微波加热下,在不添加催化剂和有机溶剂以追求绿色化学的情况下,米粉在NaCl (aq)溶液中的水解,它是食品废料中的主要成分。的NaCl的微波协同揭示通过一种创新的方法,通过跟踪和整个反应物基质同化微波实时功率输出,在血管的压力,和温度梯度的数据。使用极稀释的NaCl (水溶液)(0.05wt%)将总产物收率从23.6mol%增加到37.4mol%,其中四/三/二糖和葡萄糖是在200℃反应15分钟后的主要产物。在1wt%的NaCl (水溶液)中可获得最高的65.9mol%的产物产率。升温期间温度测量值的波动表示NaCl促进了糊化,其中反应混合物的粘度迅速变化。在13下为固体状态的核磁共振光谱表明NaCl和在C6和C2,3,5位置的游离羟基基团之间的可能的相互作用的选择性,而α(1→4)键不太课税由于空间位阻。淀粉的水解解聚由原位产生的酸物质自动催化在保温阶段。可能与糖苷键相互作用并减弱糖苷键的氯化钠的存在促进了这一步骤。从能源的角度来看,使用微波代替常规加热可将能耗降低70%,以实现相同的产品特性,因为氯化钠可通过离子损失实现出色的介电加热。这项研究为微波条件下的氯化钠-多糖相互作用和独特的氯化钠功能提供了新的见解,这是开发绿色和节能生物精炼系统的必要基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号