当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ring-opening carbonyl–olefin metathesis of norbornenes
Chemical Science ( IF 7.6 ) Pub Date : 2020-07-01 , DOI: 10.1039/d0sc02243h Janis Jermaks 1 , Phong K Quach 1 , Zara M Seibel 2 , Julien Pomarole 2 , Tristan H Lambert 1, 2
Chemical Science ( IF 7.6 ) Pub Date : 2020-07-01 , DOI: 10.1039/d0sc02243h Janis Jermaks 1 , Phong K Quach 1 , Zara M Seibel 2 , Julien Pomarole 2 , Tristan H Lambert 1, 2
Affiliation
|
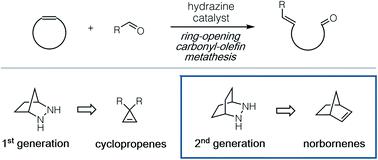
A computational and experimental study of the hydrazine-catalyzed ring-opening carbonyl–olefin metathesis of norbornenes is described. Detailed theoretical investigation of the energetic landscape for the full reaction pathway with six different hydrazines revealed several crucial aspects for the design of next-generation hydrazine catalysts. This study indicated that a [2.2.2]-bicyclic hydrazine should offer substantially increased reactivity versus the previously reported [2.2.1]-hydrazine due to a lowered activation barrier for the rate-determining cycloreversion step, a prediction which was verified experimentally. Optimized conditions for both cycloaddition and cycloreversion steps were identified, and a brief substrate scope study for each was conducted. A complication for catalysis was found to be the slow hydrolysis of the ring-opened hydrazonium intermediates, which were shown to suffer from a competitive and irreversible cycloaddition with a second equivalent of norbornene. This problem was overcome by the strategic incorporation of a bridgehead methyl group on the norbornene ring, leading to the first demonstrated catalytic carbonyl–olefin metathesis of norbornene rings.
中文翻译:

降冰片烯的开环羰基-烯烃复分解
描述了肼催化降冰片烯开环羰基-烯烃复分解反应的计算和实验研究。对六种不同肼的完整反应途径的能量景观的详细理论研究揭示了下一代肼催化剂设计的几个关键方面。这项研究表明,与之前报道的[2.2.1]-肼相比, [2.2.2]-双环肼应具有显着增加的反应性,因为其决定环化逆转步骤的活化势垒降低,这一预测已得到实验验证。确定了环加成和环化回复步骤的优化条件,并对每个步骤进行了简短的底物范围研究。人们发现催化的一个复杂问题是开环腙中间体的缓慢水解,该中间体与第二当量的降冰片烯发生竞争性且不可逆的环加成反应。通过在降冰片烯环上战略性地引入桥头甲基基团克服了这个问题,从而首次证明了降冰片烯环的催化羰基-烯烃复分解反应。
更新日期:2020-08-05
中文翻译:

降冰片烯的开环羰基-烯烃复分解
描述了肼催化降冰片烯开环羰基-烯烃复分解反应的计算和实验研究。对六种不同肼的完整反应途径的能量景观的详细理论研究揭示了下一代肼催化剂设计的几个关键方面。这项研究表明,与之前报道的[2.2.1]-肼相比, [2.2.2]-双环肼应具有显着增加的反应性,因为其决定环化逆转步骤的活化势垒降低,这一预测已得到实验验证。确定了环加成和环化回复步骤的优化条件,并对每个步骤进行了简短的底物范围研究。人们发现催化的一个复杂问题是开环腙中间体的缓慢水解,该中间体与第二当量的降冰片烯发生竞争性且不可逆的环加成反应。通过在降冰片烯环上战略性地引入桥头甲基基团克服了这个问题,从而首次证明了降冰片烯环的催化羰基-烯烃复分解反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号