当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis Development of the Selective Estrogen Receptor Degrader (SERD) LSZ102 from a Suzuki Coupling to a C–H Activation Strategy
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-07-01 , DOI: 10.1021/acs.oprd.0c00076 Markus Baenziger 1 , Marcel Baierl 1 , Krishnaswamy Devanathan 2 , Sumesh Eswaran 2 , Peng Fu 3 , Bjoern Gschwend 1 , Michael Haller 1 , Gopu Kasinathan 2 , Nikola Kovacic 1 , Audrey Langlois 1 , Yongfeng Li 4 , Friedrich Schuerch 1 , Xiaodong Shen 3 , Yinbo Wan 3 , Regina Wickendick 1 , Siwei Xie 4 , Kai Zhang 4
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-07-01 , DOI: 10.1021/acs.oprd.0c00076 Markus Baenziger 1 , Marcel Baierl 1 , Krishnaswamy Devanathan 2 , Sumesh Eswaran 2 , Peng Fu 3 , Bjoern Gschwend 1 , Michael Haller 1 , Gopu Kasinathan 2 , Nikola Kovacic 1 , Audrey Langlois 1 , Yongfeng Li 4 , Friedrich Schuerch 1 , Xiaodong Shen 3 , Yinbo Wan 3 , Regina Wickendick 1 , Siwei Xie 4 , Kai Zhang 4
Affiliation
|
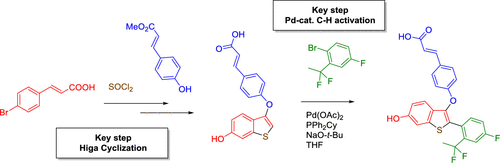
The development of the synthetic process to the selective estrogen receptor degrader (SERD) drug candidate LSZ102 from the medicinal chemistry synthesis to the streamlined large-scale manufacturing route is described. The synthesis of LSZ102 could be significantly improved in regard to overall yield, removal of all chromatographic purifications, and reduction in the number of steps by revisiting the original disconnection strategy. Key features of the final process include construction of the benzothiophene core via Higa cyclization, late-stage phenolation using a Pd-catalyzed hydroxylation of an aryl bromide, and end-game assembly through a Pd-catalyzed C–H activation step. The overall yield could be significantly improved, and the costs could be reduced.
中文翻译:

铃木偶联到C–H活化策略的选择性雌激素受体降解剂(SERD)LSZ102的合成开发
描述了从药物化学合成到精简的大规模生产路线的选择性雌激素受体降解剂(SERD)候选药物LSZ102的合成方法的发展。LSZ102的合成在总产率,所有色谱纯化方法的去除以及步骤数量的减少方面都可以通过重新考虑原始的断开策略而得到显着改善。最终工艺的关键特征包括通过Higa环化构建苯并噻吩核心,使用Pd催化的芳基溴化羟基化作用的后期酚化以及通过Pd催化的CH活化步骤进行的最终组装。可以显着提高总产量,并可以降低成本。
更新日期:2020-08-21
中文翻译:

铃木偶联到C–H活化策略的选择性雌激素受体降解剂(SERD)LSZ102的合成开发
描述了从药物化学合成到精简的大规模生产路线的选择性雌激素受体降解剂(SERD)候选药物LSZ102的合成方法的发展。LSZ102的合成在总产率,所有色谱纯化方法的去除以及步骤数量的减少方面都可以通过重新考虑原始的断开策略而得到显着改善。最终工艺的关键特征包括通过Higa环化构建苯并噻吩核心,使用Pd催化的芳基溴化羟基化作用的后期酚化以及通过Pd催化的CH活化步骤进行的最终组装。可以显着提高总产量,并可以降低成本。











































 京公网安备 11010802027423号
京公网安备 11010802027423号