当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Effect of Point Mutations on the Biophysical Properties of an Antimicrobial Peptide: Development of a Screening Protocol for Peptide Stability Screening.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-07-01 , DOI: 10.1021/acs.molpharmaceut.0c00406 Christin Pohl 1, 2 , Matja Zalar 3 , Inas El Bialy 4 , Sowmya Indrakumar 2 , Günther H J Peters 2 , Wolfgang Friess 4 , Alexander P Golovanov 3 , Werner W Streicher 1 , Allan Noergaard 1 , Pernille Harris 2
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-07-01 , DOI: 10.1021/acs.molpharmaceut.0c00406 Christin Pohl 1, 2 , Matja Zalar 3 , Inas El Bialy 4 , Sowmya Indrakumar 2 , Günther H J Peters 2 , Wolfgang Friess 4 , Alexander P Golovanov 3 , Werner W Streicher 1 , Allan Noergaard 1 , Pernille Harris 2
Affiliation
|
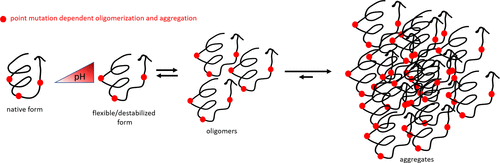
Therapeutic peptides and proteins show enormous potential in the pharmaceutical market, but high costs in discovery and development are limiting factors so far. Single or multiple point mutations are commonly introduced in protein drugs to increase their binding affinity or selectivity. They can also induce adverse properties, which might be overlooked in a functional screen, such as a decreased colloidal or thermal stability, leading to problems in later stages of the development. In this study, we address the effect of point mutations on the stability of the 4.4 kDa antimicrobial peptide plectasin, as a case study. We combined a systematic high-throughput biophysical screen of the peptide thermal and colloidal stability using dynamic light scattering and differential scanning calorimetry with structure-based methods including small-angle X-ray scattering, analytical ultracentrifugation, and nuclear magnetic resonance spectroscopy. Additionally, we applied molecular dynamics simulations to link obtained protein stability parameters to the protein’s molecular structure. Despite their predicted structural similarities, all four plectasin variants showed substantially different behavior in solution. We observed an increasing propensity of plectasin to aggregate at a higher pH, and the introduced mutations influenced the type of aggregation. Our strategy for systematically assessing the stability and aggregation of protein drugs is generally applicable and is of particular relevance, given the increasing number of protein drugs in development.
中文翻译:

点突变对抗菌肽的生物物理特性的影响:肽稳定性筛选的筛选协议的发展。
治疗性肽和蛋白质在制药市场上显示出巨大的潜力,但迄今为止,发现和开发的高成本是限制因素。单点或多点突变通常被引入蛋白质药物中以增加其结合亲和力或选择性。它们还可能引起不良特性,这些特性可能会在功能筛选中被忽略,例如胶体或热稳定性降低,从而导致开发后期出现问题。在本研究中,我们以案例研究为基础,探讨了点突变对4.4 kDa抗菌肽Plectasin稳定性的影响。我们使用动态光散射和差示扫描量热法与基于结构的方法(包括小角度X射线散射,分析超速离心和核磁共振光谱法)相结合,对肽的热和胶体稳定性进行了系统的高通量生物物理筛选。此外,我们应用分子动力学模拟将获得的蛋白质稳定性参数链接到蛋白质的分子结构。尽管它们具有预测的结构相似性,但所有四种Plectasin变体在溶液中均表现出明显不同的行为。我们观察到,在较高的pH值下,Plectasin聚集的倾向增加,并且引入的突变影响聚集的类型。
更新日期:2020-09-09
中文翻译:

点突变对抗菌肽的生物物理特性的影响:肽稳定性筛选的筛选协议的发展。
治疗性肽和蛋白质在制药市场上显示出巨大的潜力,但迄今为止,发现和开发的高成本是限制因素。单点或多点突变通常被引入蛋白质药物中以增加其结合亲和力或选择性。它们还可能引起不良特性,这些特性可能会在功能筛选中被忽略,例如胶体或热稳定性降低,从而导致开发后期出现问题。在本研究中,我们以案例研究为基础,探讨了点突变对4.4 kDa抗菌肽Plectasin稳定性的影响。我们使用动态光散射和差示扫描量热法与基于结构的方法(包括小角度X射线散射,分析超速离心和核磁共振光谱法)相结合,对肽的热和胶体稳定性进行了系统的高通量生物物理筛选。此外,我们应用分子动力学模拟将获得的蛋白质稳定性参数链接到蛋白质的分子结构。尽管它们具有预测的结构相似性,但所有四种Plectasin变体在溶液中均表现出明显不同的行为。我们观察到,在较高的pH值下,Plectasin聚集的倾向增加,并且引入的突变影响聚集的类型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号