当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamic and Kinetic Studies of the Interaction of Nuclear Receptor Coactivator-4 (NCOA4) with Human Ferritin.
Biochemistry ( IF 2.9 ) Pub Date : 2020-07-01 , DOI: 10.1021/acs.biochem.0c00246 Ayush Kumar Srivastava 1, 2 , Nicholas Flint 1 , Heidi Kreckel 1 , Magdalena Gryzik 2 , Maura Poli 2 , Paolo Arosio 2 , Fadi Bou-Abdallah 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-07-01 , DOI: 10.1021/acs.biochem.0c00246 Ayush Kumar Srivastava 1, 2 , Nicholas Flint 1 , Heidi Kreckel 1 , Magdalena Gryzik 2 , Maura Poli 2 , Paolo Arosio 2 , Fadi Bou-Abdallah 1
Affiliation
|
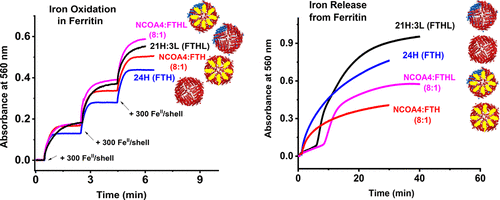
Ferritinophagy is a ferritin autophagic degradation process mediated by the selective nuclear receptor coactivator-4 (NCOA4). NCOA4 binds to ferritin and delivers it to nascent autophagosomes, which then merge with the lysosomes for ferritin degradation and iron release. Earlier studies have demonstrated a specific association of NCOA4 with ferritin H-subunits, but not L-subunits. However, neither the thermodynamics of this interaction nor the effect of NCOA4 on iron oxidation, iron mineral core formation, or iron mobilization in ferritin has been explored. Using isothermal titration calorimetry, light absorption spectroscopy, and a soluble fragment (residues 383–522) of human NCOA4 expressed in Escherichia coli, we show that the NCOA4 fragment specifically binds H-rich ferritins with a binding stoichiometry of ∼8 NCOA4 molecules per ferritin shell, and Kd values of ∼0.4 and ∼2 μM for homopolymer H-chain ferritin and heteropolymer H-rich ferritin, respectively. The binding reaction was both enthalpically and entropically favored. Whereas the iron oxidation kinetics were not affected by the presence of NCOA4, iron mobilization from ferritin by two different reducing agents (FMN/NADH and sodium dithionite) showed a strong inhibitory effect that was dependent on the concentration of NCOA4 present in solution. Our results suggest that the binding of NCOA4 to ferritin may interfere in the electron transfer pathway through the ferritin shell and may have important biological implications on cellular iron homeostasis.
中文翻译:

核受体共激活因子4(NCOA4)与人铁蛋白相互作用的热力学和动力学研究。
铁蛋白吞噬是由选择性核受体共激活因子4(NCOA4)介导的铁蛋白自噬降解过程。NCOA4结合铁蛋白并将其递送至新生的自噬体,然后与溶酶体融合以降解铁蛋白和释放铁。较早的研究表明NCOA4与铁蛋白H亚基有特定的联系,但与L亚基没有联系。但是,既没有研究这种相互作用的热力学,也没有研究NCOA4对铁氧化,铁矿物质芯形成或铁蛋白中铁动员的影响。使用等温滴定量热法,光吸收光谱法和在大肠杆菌中表达的人NCOA4的可溶性片段(残基383–522),我们显示NCOA4片段与每个富铁蛋白壳的〜8个NCOA4分子和K d的化学计量比特异性地结合了富含H的铁蛋白均聚物H链铁蛋白和杂聚物富含H的铁蛋白的分别约为0.4和2μM。结合反应在焓和熵上都是有利的。尽管铁的氧化动力学不受NCOA4的存在的影响,但铁蛋白通过两种不同的还原剂(FMN / NADH和连二亚硫酸钠)从铁蛋白中迁移出来的铁显示出强大的抑制作用,这取决于溶液中NCOA4的浓度。我们的结果表明,NCOA4与铁蛋白的结合可能会干扰通过铁蛋白壳的电子转移途径,并且可能对细胞铁稳态具有重要的生物学意义。
更新日期:2020-07-28
中文翻译:

核受体共激活因子4(NCOA4)与人铁蛋白相互作用的热力学和动力学研究。
铁蛋白吞噬是由选择性核受体共激活因子4(NCOA4)介导的铁蛋白自噬降解过程。NCOA4结合铁蛋白并将其递送至新生的自噬体,然后与溶酶体融合以降解铁蛋白和释放铁。较早的研究表明NCOA4与铁蛋白H亚基有特定的联系,但与L亚基没有联系。但是,既没有研究这种相互作用的热力学,也没有研究NCOA4对铁氧化,铁矿物质芯形成或铁蛋白中铁动员的影响。使用等温滴定量热法,光吸收光谱法和在大肠杆菌中表达的人NCOA4的可溶性片段(残基383–522),我们显示NCOA4片段与每个富铁蛋白壳的〜8个NCOA4分子和K d的化学计量比特异性地结合了富含H的铁蛋白均聚物H链铁蛋白和杂聚物富含H的铁蛋白的分别约为0.4和2μM。结合反应在焓和熵上都是有利的。尽管铁的氧化动力学不受NCOA4的存在的影响,但铁蛋白通过两种不同的还原剂(FMN / NADH和连二亚硫酸钠)从铁蛋白中迁移出来的铁显示出强大的抑制作用,这取决于溶液中NCOA4的浓度。我们的结果表明,NCOA4与铁蛋白的结合可能会干扰通过铁蛋白壳的电子转移途径,并且可能对细胞铁稳态具有重要的生物学意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号