当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantitative Analysis of Redox-Inactive Ions by AC Voltammetry at a Polarized Interface between Two Immiscible Electrolyte Solutions.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-07-01 , DOI: 10.1021/acs.analchem.0c01340 Marco F Suárez-Herrera 1 , Micheál D Scanlon 2
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-07-01 , DOI: 10.1021/acs.analchem.0c01340 Marco F Suárez-Herrera 1 , Micheál D Scanlon 2
Affiliation
|
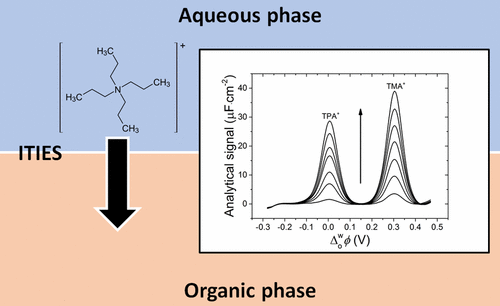
The interface between two immiscible electrolyte solutions (ITIES) is ideally suited to detect redox-inactive ions by their ion transfer. Such electroanalysis, based on the Nernst–Donnan equation, has been predominantly performed using amperometry, cyclic voltammetry, or differential pulse voltammetry. Here, we introduce a new electroanalytical method based on alternating-current (AC) voltammetry with inherent advantages over traditional approaches such as avoidance of positive feedback iR compensation, a major issue for liquid|liquid electrochemical cells containing resistive organic media and interfacial areas in the cm2 and mm2 range. A theoretical background outlining the generation of the analytical signal is provided and based on extracting the component that depends on the Warburg impedance from the total impedance. The quantitative detection of a series of model redox-inactive tetraalkylammonium cations is demonstrated, with evidence provided of the transient adsorption of these cations at the interface during the course of ion transfer. Since ion transfer is diffusion-limited, by changing the voltage excitation frequency during AC voltammetry, the intensity of the Faradaic response can be enhanced at low frequencies (1 Hz) or made to disappear completely at higher frequencies (99 Hz). The latter produces an AC voltammogram equivalent to a “blank” measurement in the absence of analyte and is ideal for background subtraction. Therefore, major opportunities exist for the sensitive detection of ionic analyte when a “blank” measurement in the absence of analyte is impossible. This approach is particularly useful to deconvolute signals related to reversible electrochemical reactions from those due to irreversible processes, which do not give AC signals.
中文翻译:

通过交流伏安法在两个不混溶的电解质溶液之间的极化界面上定量分析氧化还原惰性离子。
两种不混溶的电解质溶液(ITIES)之间的界面非常适合通过其离子转移来检测氧化还原惰性离子。这种基于Nernst-Donnan方程的电分析主要使用安培法,循环伏安法或微分脉冲伏安法进行。在此,我们介绍一种基于交流(AC)伏安法的新电分析方法,该方法具有优于传统方法的固有优势,例如避免了正反馈iR补偿,对于包含电阻性有机介质和界面区域的液体电化学电池的主要问题。 cm 2和mm 2范围。提供了理论背景,概述了分析信号的生成,并且基于从总阻抗中提取依赖于Warburg阻抗的分量。证明了对一系列模型氧化还原无活性四烷基铵阳离子的定量检测,并提供了在离子转移过程中这些阳离子在界面上的瞬时吸附的证据。由于离子传递受到扩散限制,因此通过在交流伏安法期间更改电压激励频率,可以在低频(1 Hz)时增强法拉第响应的强度,或者在高频(99 Hz)时完全消失。在没有分析物的情况下,后者产生的交流伏安图等效于“空白”测量值,是背景扣除的理想选择。因此,当不可能在没有分析物的情况下进行“空白”测量时,存在灵敏检测离子分析物的主要机会。这种方法对于将与可逆电化学反应有关的信号与由于不可逆过程而产生的不可逆过程的信号反卷积特别有用,该过程不会产生AC信号。
更新日期:2020-08-04
中文翻译:

通过交流伏安法在两个不混溶的电解质溶液之间的极化界面上定量分析氧化还原惰性离子。
两种不混溶的电解质溶液(ITIES)之间的界面非常适合通过其离子转移来检测氧化还原惰性离子。这种基于Nernst-Donnan方程的电分析主要使用安培法,循环伏安法或微分脉冲伏安法进行。在此,我们介绍一种基于交流(AC)伏安法的新电分析方法,该方法具有优于传统方法的固有优势,例如避免了正反馈iR补偿,对于包含电阻性有机介质和界面区域的液体电化学电池的主要问题。 cm 2和mm 2范围。提供了理论背景,概述了分析信号的生成,并且基于从总阻抗中提取依赖于Warburg阻抗的分量。证明了对一系列模型氧化还原无活性四烷基铵阳离子的定量检测,并提供了在离子转移过程中这些阳离子在界面上的瞬时吸附的证据。由于离子传递受到扩散限制,因此通过在交流伏安法期间更改电压激励频率,可以在低频(1 Hz)时增强法拉第响应的强度,或者在高频(99 Hz)时完全消失。在没有分析物的情况下,后者产生的交流伏安图等效于“空白”测量值,是背景扣除的理想选择。因此,当不可能在没有分析物的情况下进行“空白”测量时,存在灵敏检测离子分析物的主要机会。这种方法对于将与可逆电化学反应有关的信号与由于不可逆过程而产生的不可逆过程的信号反卷积特别有用,该过程不会产生AC信号。











































 京公网安备 11010802027423号
京公网安备 11010802027423号