当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Yeast Intracellular Staining (yICS): Enabling High-Throughput, Quantitative Detection of Intracellular Proteins via Flow Cytometry for Pathway Engineering.
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-06-30 , DOI: 10.1021/acssynbio.0c00199 Brett D Hill 1 , Ponnandy Prabhu 1 , Syed M Rizvi 1 , Fei Wen 1
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-06-30 , DOI: 10.1021/acssynbio.0c00199 Brett D Hill 1 , Ponnandy Prabhu 1 , Syed M Rizvi 1 , Fei Wen 1
Affiliation
|
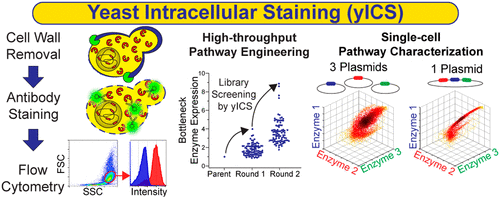
The complexities of pathway engineering necessitate screening libraries to discover phenotypes of interest. However, this approach is challenging when desirable phenotypes cannot be directly linked to growth advantages or fluorescence. In these cases, the ability to rapidly quantify intracellular proteins in the pathway of interest is critical to expedite the clonal selection process. While Saccharomyces cerevisiae remains a common host for pathway engineering, current approaches for intracellular protein detection in yeast either have low throughput, can interfere with protein function, or lack the ability to detect multiple proteins simultaneously. To fill this need, we developed yeast intracellular staining (yICS) that enables fluorescent antibodies to access intracellular compartments of yeast cells while maintaining their cellular integrity for analysis by flow cytometry. Using the housekeeping proteins β actin and glyceraldehyde 3-phophate dehydrogenase (GAPDH) as targets for yICS, we demonstrated for the first time successful antibody-based flow cytometric detection of yeast intracellular proteins with no modification. Further, yICS characterization of a recombinant d-xylose assimilation pathway showed 3-plexed, quantitative detection of the xylose reductase (XR), xylitol dehydrogenase (XDH), and xylulokinase (XK) enzymes each fused with a small (6–10 amino acids) tag, revealing distinct enzyme expression profiles between plasmid-based and genome-integrated expression approaches. As a result of its high-throughput and quantitative capability, yICS enabled rapid screening of a library created from CRISPR-mediated XDH integration into the yeast δ site, identifying rare (1%) clones that led to an 8.4-fold increase in XDH activity. These results demonstrate the utility of yICS for greatly accelerating pathway engineering efforts, as well as any application where the high-throughput and quantitative detection of intracellular proteins is desired.
中文翻译:

酵母细胞内染色(yICS):通过流式细胞术对途径工程进行高通量,定量检测细胞内蛋白质。
途径工程的复杂性需要筛选文库以发现感兴趣的表型。然而,当所需的表型不能直接与生长优势或荧光联系起来时,这种方法就具有挑战性。在这些情况下,快速量化目标途径中细胞内蛋白质的能力对于加快克隆选择过程至关重要。虽然酿酒酵母仍然是途径工程的常见宿主,目前酵母中细胞内蛋白质检测的方法要么通量低,可能干扰蛋白质功能,要么缺乏同时检测多种蛋白质的能力。为了满足这一需求,我们开发了酵母细胞内染色(yICS),使荧光抗体能够进入酵母细胞的细胞内区室,同时保持其细胞完整性,以便通过流式细胞仪进行分析。使用管家蛋白β肌动蛋白和3-磷酸甘油醛脱氢酶(GAPDH)作为yICS的靶标,我们首次证明了无需修饰即可成功基于抗体的流式细胞术检测酵母细胞内蛋白。此外,重组d的yICS表征-木糖同化途径对木糖还原酶(XR),木糖醇脱氢酶(XDH)和木酮糖激酶(XK)酶进行了3重定量检测,每种酶都融合了小的(6-10个氨基酸)标签,揭示了不同的酶表达谱基于质粒和基因组整合的表达方法之间的关系。由于其高通量和定量能力,yICS能够快速筛选由CRISPR介导的XDH整合入酵母δ位点而创建的文库,从而鉴定出导致XDH活性提高8.4倍的稀有(1%)克隆。 。这些结果证明了yICS在大大加速途径工程工作以及需要高通量和定量检测细胞内蛋白的任何应用中的实用性。
更新日期:2020-08-21
中文翻译:

酵母细胞内染色(yICS):通过流式细胞术对途径工程进行高通量,定量检测细胞内蛋白质。
途径工程的复杂性需要筛选文库以发现感兴趣的表型。然而,当所需的表型不能直接与生长优势或荧光联系起来时,这种方法就具有挑战性。在这些情况下,快速量化目标途径中细胞内蛋白质的能力对于加快克隆选择过程至关重要。虽然酿酒酵母仍然是途径工程的常见宿主,目前酵母中细胞内蛋白质检测的方法要么通量低,可能干扰蛋白质功能,要么缺乏同时检测多种蛋白质的能力。为了满足这一需求,我们开发了酵母细胞内染色(yICS),使荧光抗体能够进入酵母细胞的细胞内区室,同时保持其细胞完整性,以便通过流式细胞仪进行分析。使用管家蛋白β肌动蛋白和3-磷酸甘油醛脱氢酶(GAPDH)作为yICS的靶标,我们首次证明了无需修饰即可成功基于抗体的流式细胞术检测酵母细胞内蛋白。此外,重组d的yICS表征-木糖同化途径对木糖还原酶(XR),木糖醇脱氢酶(XDH)和木酮糖激酶(XK)酶进行了3重定量检测,每种酶都融合了小的(6-10个氨基酸)标签,揭示了不同的酶表达谱基于质粒和基因组整合的表达方法之间的关系。由于其高通量和定量能力,yICS能够快速筛选由CRISPR介导的XDH整合入酵母δ位点而创建的文库,从而鉴定出导致XDH活性提高8.4倍的稀有(1%)克隆。 。这些结果证明了yICS在大大加速途径工程工作以及需要高通量和定量检测细胞内蛋白的任何应用中的实用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号