当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Approach to the Core Structure of Streptosetin A
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-07-01 , DOI: 10.1002/slct.202001018 Stefan Hess 1 , Martin E. Maier 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-07-01 , DOI: 10.1002/slct.202001018 Stefan Hess 1 , Martin E. Maier 1
Affiliation
|
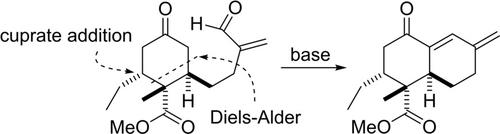
Streptosetin A belongs to the 3‐decalinoyltetramic acids. In contrast to most of other known natural products of this type, the decalin part features a β‐hydroxyketone subunit, making an intramolecular cycloaddition approach less suitable. We examined an approach where the decalin part would be fashioned by an intramolecular aldol addition. By using a Diels‐Alder reaction between the Rawal diene and a substituted methacrylate, a cyclohexanone was obtained. An organocuprate addition introduced the ethyl substituent before, the side chain was converted to an enal. However, contrary to our expectations, the aldol reaction led to the condensation product. Other routes to reach the key cyclohexanone were also investigated.
中文翻译:

链霉菌素A核心结构的探讨
链霉菌素A属于3-decalinoyltetramic酸。与大多数其他已知的此类天然产物相比,十氢化萘部分具有β-羟基酮亚基,因此分子内环加成法不太合适。我们研究了一种方法,其中萘烷部分将通过分子内羟醛的添加形成。通过在拉瓦尔二烯和取代的甲基丙烯酸酯之间进行Diels-Alder反应,获得了环己酮。在将有机取代基引入乙基取代基之前,侧链被转化为烯醛。但是,与我们的预期相反,醛醇缩合反应导致了缩合产物的产生。还研究了到达关键环己酮的其他途径。
更新日期:2020-07-01
中文翻译:

链霉菌素A核心结构的探讨
链霉菌素A属于3-decalinoyltetramic酸。与大多数其他已知的此类天然产物相比,十氢化萘部分具有β-羟基酮亚基,因此分子内环加成法不太合适。我们研究了一种方法,其中萘烷部分将通过分子内羟醛的添加形成。通过在拉瓦尔二烯和取代的甲基丙烯酸酯之间进行Diels-Alder反应,获得了环己酮。在将有机取代基引入乙基取代基之前,侧链被转化为烯醛。但是,与我们的预期相反,醛醇缩合反应导致了缩合产物的产生。还研究了到达关键环己酮的其他途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号